Brand Names: Various around the world
What is abacavir?
Antiviral medication abacavir is prescribed for the treatment of infection caused by the human immunodeficiency virus (HIV). Abacavir-containing brand names such as Ziagen, Kivexa, Triumeq, Trizivir, and Epzicom are well-known. Several medical journals and research papers pertaining to the specific active constituent abacavir will be analysed in this article, with less emphasis on the various brand names.
HIV infection is treated with abacavir, a nucleoside reverse transcriptase inhibitor (NRTI), in combination with other antiretroviral drugs. It functions by impeding the activity of reverse transcriptase, an enzyme that is vital for the process of HIV replication within the organism. Through the inhibition of viral replication, abacavir aids in the mitigation of viral burden within the body and enhances immune system functionality among individuals living with HIV.
Therapeutic category
It is classified as an antiretroviral medication within the therapeutic category, more specifically as a nucleoside reverse transcriptase inhibitor (NRTI). NRTIs are a class of antiretroviral pharmaceuticals that, when combined with other NRTIs, inhibit the reverse transcriptase enzyme’s activity in order to treat HIV infection.
Chemical structure and properties
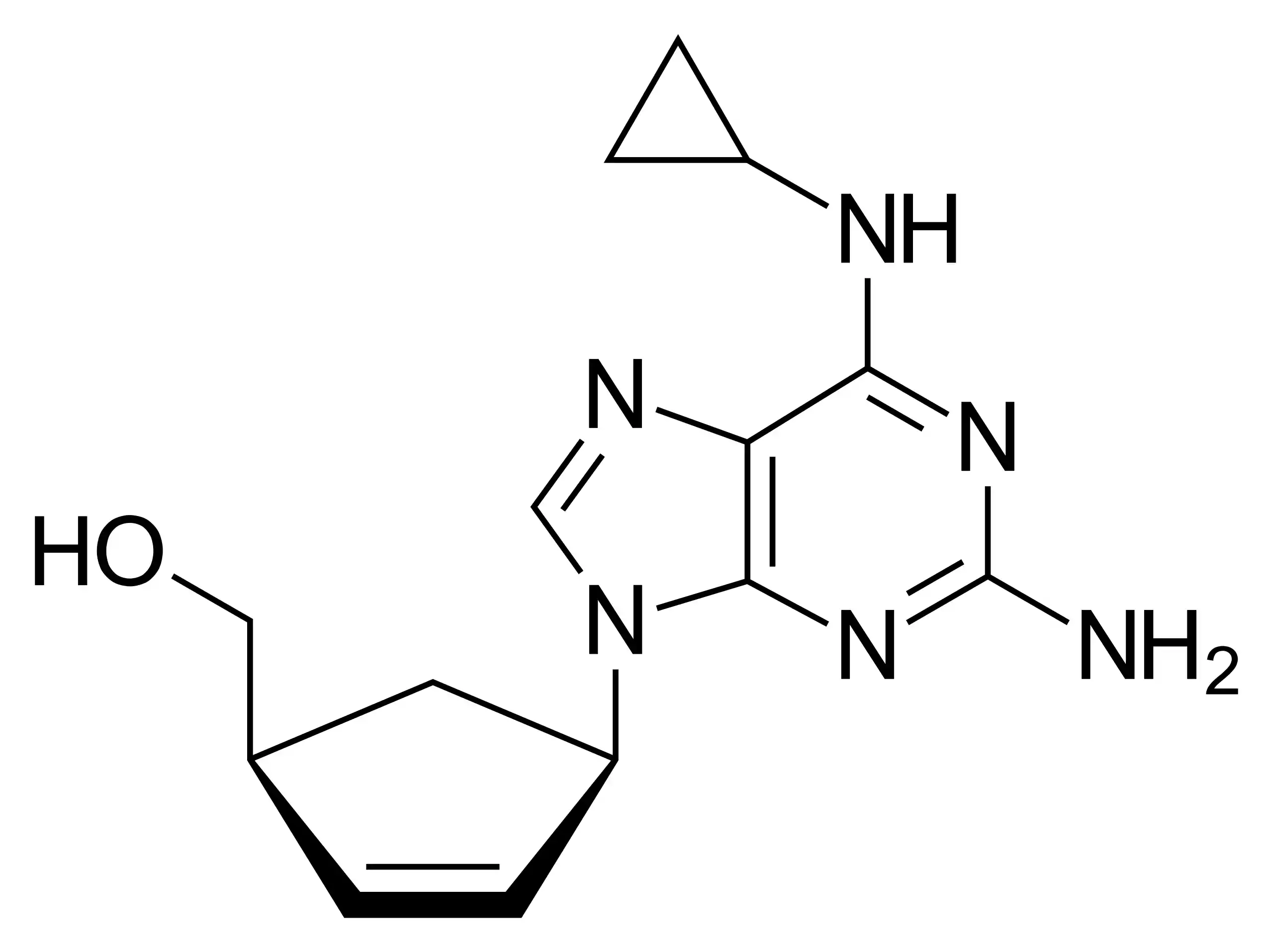
The chemical formula for abacavir, a synthetic carbocyclic nucleoside analogue, is C14H18N6O. It is a water-soluble crystalline solid ranging in colour from white to off-white. It possesses a molecular weight of 286.332 g/mol. It possesses a chemical structure comparable to that of the naturally occurring nucleoside guanosine.
Mechanism of action
It undergoes intracellular phosphorylation to yield carbovir triphosphate, its active metabolite. This metabolite vies with deoxyguanosine triphosphate, the natural substrate, for integration into viral DNA via HIV reverse transcriptase. Abacavir triphosphate prevents viral replication and impedes the activity of HIV reverse transcriptase through its incorporation into the viral DNA, which results in chain termination (Walmsley et al., 2013).
Indications for use
It is prescribed in conjunction with other antiretroviral medications for the treatment of HIV infection. It is administered in conjunction with other medications to treat HIV infection in both infants and adults. While abacavir does not offer a curative effect for HIV infection, it does possess the potential to mitigate viral load and enhance immune system functionality. These effects collectively contribute to an improved quality of life for individuals living with HIV and a decreased risk of HIV-related complications.
Warnings
Precautions before taking the drug
Patients who are going to start taking it should be checked for the HLA-B*5701 gene because this allele is linked to a much higher risk of hypersensitivity responses (HSRs) to abacavir (Cruciani et al.). People who have had HSRs to abacavir or other NRTIs in the past should not be given treatments that contain abacavir. Check the kidney and liver performance before starting it. People whose kidneys or livers don’t work well may need to change their dose.
Contraindications
It should not be taken by people who are known to be allergic to it or any of its ingredients. People who have mild to serious liver damage shouldn’t use it because it could make them more likely to get lactic acidosis and liver damage. We do not suggest giving abacavir with other NRTIs, like emtricitabine or lamivudine, because the side effects could be worse.
What you should avoid
People who are taking it shouldn’t drink alcohol because it may raise the risk of hepatotoxicity. When taking it with other drugs that could hurt the liver or mess up the digestion of abacavir, users should be very careful. Before starting abacavir treatment, patients should tell their doctor about all of the medicines, vitamins, and herbal products they are currently using. This will help lower the risk of drug interactions.
Dosage and administration
It should be taken by adults every day. It can be split into two doses of 300 mg each, or it can be taken all at once. The right amount for kids depends on their weight. For kids under 14 kg, the advice is 8 mg/kg twice a day, and for kids 14 kg and up, it’s 16 mg/kg once a day, up to a maximum of 600 mg a day (Walmsley et al.).
Routes and methods of administration
You can get it in the form of pills or oral treatments. It’s okay to take the pills with or without food. You can swallow them whole with water. Use the dose syringe that comes with the oral solution to measure it out. To make it taste better, mix it with a little water or juice. Patients should be told to take abacavir at the same time every day so that therapeutic levels stay stable and patients don’t miss doses or take two of the ones they missed.
What should I do if I miss a dose?
Patients who forget to take their dose should do so as soon as they remember, as long as it’s not less than two hours before their next dose. In this case, the patient should not take the missed dose but instead go back to their normal dosing schedule. Patients shouldn’t take two doses at once to make up for one they missed, as this may raise the risk of side effects. Sticking to the recommended dosing schedule is important to get the most out of the treatment and stop the virus from becoming resistant (Bedimo et al.).
If a person thinks they may have taken too much abacavir, they should get medical help right away. Some signs of an overdose are feeling sick, throwing up, having diarrhoea, and stomach pain. When someone takes too much, they are mostly given supporting care while their vital signs, electrolyte levels, and liver function are closely watched. People with severe cases might want to try haemodialysis, which can remove some of it.
Side Effects
The most common side effects of taking it are feeling sick, throwing up, having diarrhoea, being tired, getting headaches, and having trouble sleeping. Most of the time, these effects are mild to moderate, and they go away as the treatment continues. But it can also lead to very bad side effects, like hypersensitivity responses (HSRs) and lactic acidosis. Most of the time, HSRs happen in the first six weeks of treatment. They can show up as fever, rash, stomach problems, or trouble breathing. If you think a patient has a HSR, you should stop giving them abacavir right away and not give it to them again because the reactions could be serious and even life-threatening (Cruciani et al.).
Interactions
Drug-drug interactions
Abacavir might change how well other medicines work or make side effects more likely when taken with them. It is best not to take it with booze or other drugs that could hurt your liver, because this could increase the risk of hepatotoxicity. It may also interact with methadone, lowering the amount of drug in the blood and possibly causing opioid withdrawal signs (Walmsley et al.). Before starting abacavir therapy, patients should tell their doctor about all the medicines they are taking to lower the chance of drug interactions.
Drug-food interactions
There are no known drug-food combinations that are clinically important for abacavir. You can take the medicine with or without food, but if you have stomach problems, taking it with food might help. Patients taking abacavir should eat a healthy, well-balanced diet to improve their general health and well-being.
Additional important information
Special warnings for the elderly and children
Patients who are older or who already have problems with their kidneys or liver may be more likely to have bad reactions to it. Patients over 65 whose kidneys aren’t working as well may need to have their doses changed. Children ages 3 months and up have been shown to be safe and effective when given abacavir. Children, on the other hand, need to be closely watched because they may be more likely to have hypersensitivity responses (HSRs) and other bad things happen (Walmsley et al.).
Development of resistance
Resistance to it can happen, especially if the drug is used alone or in a way that isn’t ideal. Choosing certain changes in the reverse transcriptase gene, like M184V and K65R, makes it resistant to abacavir. Always take it with other antiretroviral drugs to lower the chance of resistance, and make sure you follow the directions exactly (Cruciani et al.).
Clinical studies
A lot of clinical studies have looked into how well and safely it treats HIV-1 illness. The ACTG 5202 study looked at how well abacavir/lamivudine, tenofovir/emtricitabine, and either atazanavir/ritonavir or efavirenz were working together in people who had never been on treatment for HIV. It was found that people who took tenofovir/emtricitabine were less likely to have HSRs and their viruses failed than people who took abacavir/lamivudine.
Comparative effectiveness
The effectiveness and safety of abacavir have been compared to those of other NRTIs by researchers. A study by Cruciani et al. looked at many studies and found that regimens with it had a higher chance of failing than regimens with tenofovir. This was especially true for people who had a lot of bugs to begin with. However, abacavir-containing regimens worked just as well as other NRTI-based regimens in people who already had low viral loads.
The pharmacological features
When taken by mouth, it is quickly absorbed, and about 83% of it is bioavailable. The liver breaks down the drug a lot with the help of alcohol dehydrogenase and glucuronyl transferase. The potent substance, carbovir triphosphate, has a half-life inside cells of 20 to 24 hours, which means that it can only be taken once a day. About 2% of the dose of abacavir is passed out of the body intact in the urine (Walmsley et al.).
Briefly
When taken in conjunction with other medications, the antiretroviral medicine abacavir is used to treat HIV infection. It functions by preventing reverse transcriptase, an enzyme essential for HIV replication, from doing its job. It is a member of the nucleoside reverse transcriptase inhibitor (NRTI) class. Its active metabolite, carbovir triphosphate, is produced intracellularly. It ends the elongation of the viral DNA chain by competing with its natural substrate, deoxyguanosine triphosphate. Patients should have an HLA-B*5701 allele screening prior to starting treatment, since this allele is linked to a higher risk of hypersensitivity responses. Nausea, vomiting, diarrhoea, exhaustion, headaches, and sleeplessness are typical adverse effects. Serious side effects are also possible, including lactic acidosis and hypersensitivity reactions. Patients with hepatic impairment should use it cautiously since it may interact with other drugs, requiring dose modifications or alternate treatment options. It is important to adhere to the specified combination medication as resistance to it might arise, especially when administered as solo or in suboptimal regimens.
ATTENTION: It is of vital importance to never take any medication without the supervision and guidance of a specialised doctor. Consult the package insert of each prescribed medicinal product, as each pharmaceutical company accurately describes the specific specifications for the product, which may undergo regular updates. Note that the trade names mentioned in this article correspond to well-known medicinal products that contain the active substances under analysis. However, there may be variations depending on the composition of each drug. This article focuses on the active substance analysis rather than the drug’s trade name. The reference to trade names is made exclusively for the convenience of readers, who should carefully study the instruction leaflet for each commercial preparation they use. It is necessary to have close cooperation with your attending physician and your pharmacist. The self-administration of any medication carries serious health risks and should be strictly avoided.
Bibliography
- Walmsley, S. L., Antela, A., Clumeck, N., Duiculescu, D., Eberhard, A., Gutiérrez, F., … & Stalker, S. (2013). Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. New England Journal of Medicine, 369(19), 1807-1818. nejm
- Cruciani, M., Mengoli, C., Malena, M., Serpelloni, G., Parisi, S. G., & Bosco, O. (2014). Virological efficacy of abacavir: systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy, 69(12), 3169-3180. academic.oup
- Bedimo, R. J., Westfall, A. O., Drechsler, H., Vidiella, G., & Tebas, P. (2011). Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clinical Infectious Diseases, 53(1), 84-91. academic.oup


