Table of Contents
ToggleGeneric Name: Dabrafenib
Brand Names: Various around the world
What is Dabrafenib?
Dabrafenib is an oral medication used to treat certain types of cancer, particularly melanoma that has spread to other parts of the body or cannot be removed by surgery. Dabrafenib side effects will be extensively analysed later in this text. Some popular brand names containing the generic medicine dabrafenib include Tafinlar, Tafidab, and Dabfna. Dabrafenib was discovered and developed by GlaxoSmithKline, receiving approval from the U.S. Food and Drug Administration (FDA) in 2013 for the treatment of metastatic melanoma with BRAF V600E mutation. This article will analyse several medical research papers and journals concerning the specific active ingredient dabrafenib, such as “Dabrafenib and its potential for the treatment of metastatic melanoma” by R Kainthla, KB Kim, and GS Falchook, published in Small Molecules in Oncology (2014), and “Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial” by AM Menzies, GV Long, and R Murali, published in Drug Design, Development and Therapy (2012).
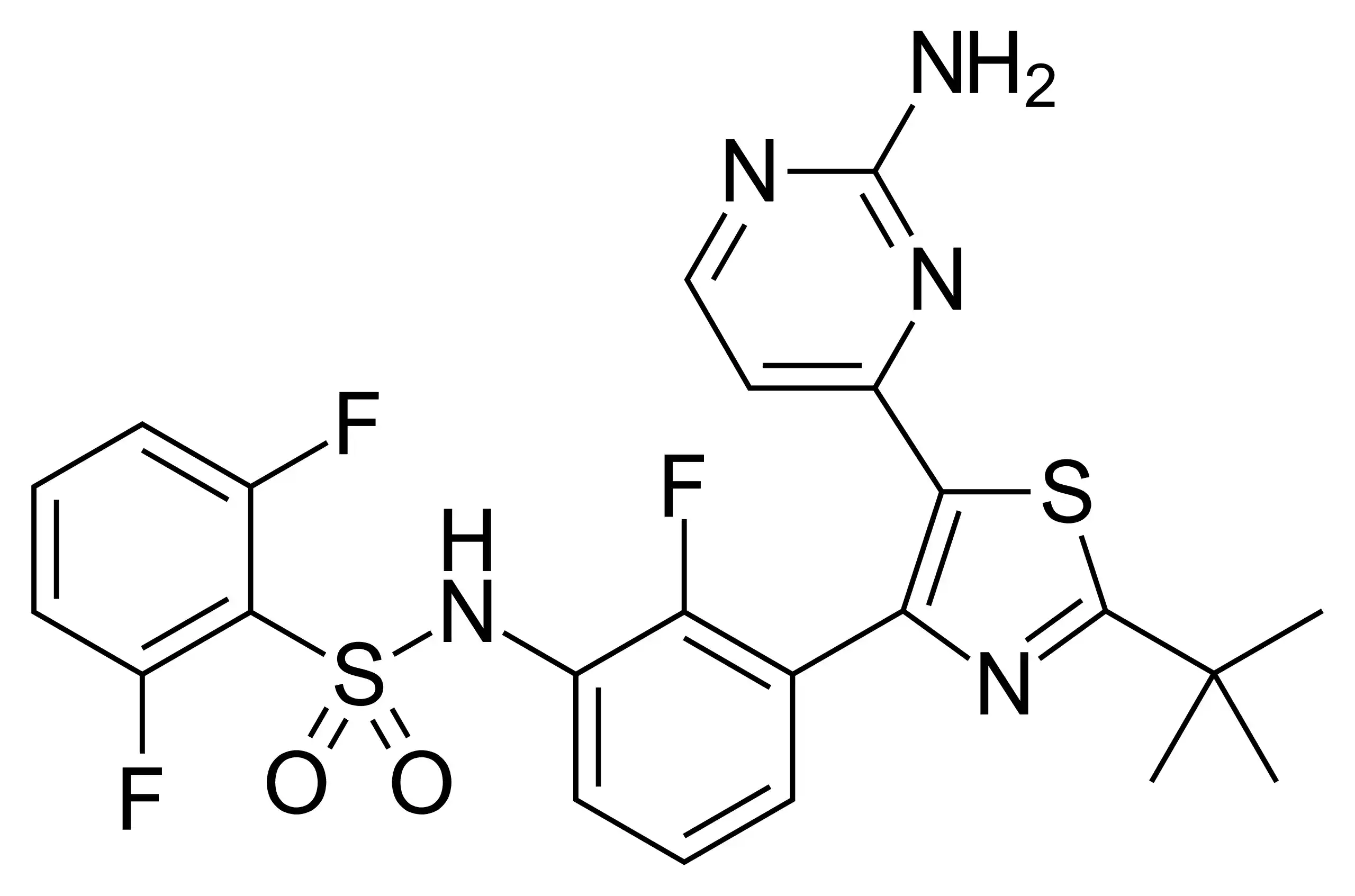
Chemical Structure and Mechanism of Action
Dabrafenib, chemically known as N-{3-[5-(2-aminopyrimidin-4-yl)-2-(tert-butyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide, is a selective inhibitor of the BRAF V600E mutant kinase (Kainthla et al., 2014). The BRAF gene is responsible for producing a protein called B-Raf, which is involved in sending signals within cells and regulating cell growth. In about half of all melanomas, the BRAF gene is mutated, leading to uncontrolled cell growth and tumour formation.
Dabrafenib works by blocking the activity of the mutated B-Raf protein, thereby slowing or stopping the growth of cancer cells. It binds to the ATP-binding site of BRAF V600E, preventing ATP from binding and activating the kinase (Menzies et al., 2012). By selectively targeting the mutant form of BRAF, dabrafenib minimizes the impact on normal, healthy cells. The specific targeting of mutant BRAF is crucial, as inhibition of wild-type BRAF can lead to paradoxical activation of the MAPK pathway and potentially stimulate tumour growth (Falchook et al., 2012). Dabrafenib’s mechanism of action makes it an effective targeted therapy for patients with BRAF V600E-mutated melanoma, offering a more personalized approach to cancer treatment.
Uses
Dabrafenib is primarily prescribed for the treatment of unresectable or metastatic melanoma with a BRAF V600E mutation, as confirmed by an FDA-approved test. One kind of skin cancer that appears in the melanocytes—the cells that produce the pigment that gives skin its color—is called melanoma. Melanoma is categorised as metastatic or unresectable depending on whether it spreads to other body areas or cannot be surgically removed. A particular mutation in the BRAF gene called V600E causes unchecked proliferation of cancer cells in around half of melanoma cases (Kainthla et al.).
Numerous clinical studies have shown that dabrafenib is an effective treatment for melanoma with a BRAF V600E mutation. Dabrafenib showed encouraging anticancer efficacy in patients with untreated brain metastases, various solid tumours, including melanoma in a phase 1 dose-escalation experiment (Menzies et al.). Dabrafenib was shown to be well-tolerated and to have a high response rate in patients, including those with brain metastases, who had melanoma with a BRAF V600E mutation.
Apart from its single-agent use, dabrafenib is also combined with trametinib, an additional targeted therapeutic that impedes the MEK protein, a component of the same signalling pathway as BRAF. When compared to dabrafenib alone, the combination of trametinib with dabrafenib has been demonstrated to enhance both overall survival and progression-free survival in patients with metastatic melanoma mutated in the BRAF gene.
Dabrafenib has been studied for the treatment of additional solid tumours carrying the BRAF V600E mutation, such as thyroid cancer and non-small cell lung cancer (NSCLC), in addition to its original use for metastatic melanoma. Although dabrafenib has shown promise in treating certain tumours in certain trials, further investigation is required to fully understand this medication’s function in treating these illnesses.
To find out if dabrafenib is the right medication for their particular illness, people must speak with their healthcare professional. When choosing a course of therapy, one should take into account several factors, including the patient’s medical history and general health, the stage and extent of the disease, and the existence of the BRAF V600E mutation.
Dabrafenib Side Effects
As with any medication, dabrafenib can cause side effects, ranging from common and manageable to rare but potentially serious. It is crucial for patients to be aware of these side effects and to report any unusual symptoms to their healthcare provider promptly.
Common Dabrafenib Side Effects
The most common side effects associated with dabrafenib include fatigue, headache, nausea, vomiting, diarrhoea, constipation, hair loss, and skin-related issues such as rash, dry skin, itching, and redness. These side effects are generally mild to moderate in severity and can often be managed with supportive care or by adjusting the dosage under the guidance of a healthcare professional. In a phase 1 dose-escalation trial, the most frequently reported adverse events were skin-related, including hyperkeratosis, papillomas, and palmar-plantar erythrodysesthesia (Falchook et al.).
Rare but Possible Dabrafenib Side Effects
Some less common but potentially concerning side effects of dabrafenib include blurred vision, eye pain or redness, fever, chills, persistent cough, difficulty breathing, irregular heartbeat, swelling of the face, lips, tongue, or throat, and severe skin reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis. These side effects may require prompt medical attention and should be reported to a healthcare provider immediately.
Serious Dabrafenib Side Effects
In rare cases, dabrafenib can cause serious side effects that may be life-threatening. These include the development of new primary cutaneous malignancies, such as squamous cell carcinoma, keratoacanthoma, or melanoma. Regular skin examinations are recommended for patients taking dabrafenib to detect any new skin lesions early (Kainthla et al.). Other serious side effects may include severe febrile reactions, hyperglycemia, uveitis, and hemolytic anemia. Patients should be closely monitored for signs and symptoms of these serious side effects, and treatment should be discontinued if they occur.
It is important to note that the side effects experienced by patients may vary depending on individual factors such as age, overall health, and the presence of other medical conditions. Patients should maintain open communication with their healthcare provider throughout their treatment with dabrafenib to ensure that any side effects are promptly addressed and managed appropriately. In some cases, dose adjustments or temporary treatment interruptions may be necessary to mitigate the impact of side effects while still maintaining the efficacy of the treatment.
Warnings
Before starting dabrafenib treatment, patients need to know about a few crucial risks and warnings. First off, individuals who have had the BRAF V600E mutation verified by an FDA-approved test need to be the only ones offered dabrafenib. According to Menzies et al., dabrafenib usage in individuals without this particular mutation may not only be ineffective but also have the potential to accelerate the growth of the tumour.
This drug should not be taken by patients who have previously experienced allergic reactions to dabrafenib or any of its inactive components. Furthermore, dabrafenib given to pregnant women may damage the foetus. As recommended by their healthcare professional, women who are capable of becoming pregnant should take an effective form of contraception both throughout and after the final dosage of therapy.
Serious fever responses brought on by dabrafenib may also include chills, rigours, hypotension, dehydration, and renal failure. Hospitalisation may be necessary in certain circumstances due to these responses. Patients should be urged to report any fever or odd symptoms to their healthcare practitioner as soon as they occur, and they should be regularly watched for pyrexia signs and symptoms (Kainthla et al.).
A higher chance of developing new primary cutaneous malignancies, such as squamous cell carcinoma, keratoacanthoma, or melanoma, has been linked to the use of dabrafenib. To identify any new skin lesions as soon as possible, patients should have routine skin exams done prior to, during, and following dabrafenib therapy. It is important to examine suspicious lesions as away and treat them if needed.
Additionally, dabrafenib may result in hyperglycemia, especially in people who already have diabetes or who are taking other drugs that may impact glucose metabolism. Regular blood glucose monitoring is recommended, and if hyperglycemia develops, suitable treatment measures should be put in place.
Additional cautions related to dabrafenib include the possibility of medication interactions, hemolytic anaemia, and uveitis. Before beginning dabrafenib treatment, patients should disclose to their healthcare practitioner any drugs, vitamins, and herbal items they are using. Certain drugs, such strong CYP3A4 inducers or inhibitors, may alter dabrafenib plasma concentrations and necessitate dosage modifications or avoidance.
Patients should be informed that dabrafenib can make skin more sensitive to sunlight, so they should avoid extended sun exposure and use protective clothes and broad-spectrum sunscreen when they are outside. To check for the emergence of uveitis or other ocular toxicities, routine eye exams are also advised (Falchook et al.).
Precautions
Prior to commencing treatment with dabrafenib, several precautionary measures should be considered. Patients with a history of pancreatic or biliary tract disease should be closely monitored, as dabrafenib has been associated with an increased risk of pancreatitis and biliary tract disorders. Renal function should also be assessed before and during treatment, as dabrafenib may cause renal impairment, particularly in patients with pre-existing renal dysfunction. Dose adjustments or discontinuation may be necessary in cases of severe renal impairment (Menzies et al., “Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial”).
Patients with a history of cardiovascular disease or risk factors for cardiovascular events should undergo a comprehensive cardiac evaluation prior to initiating dabrafenib. This medication may prolong the QT interval and increase the risk of ventricular arrhythmias, particularly in patients with congenital long QT syndrome or those taking concomitant medications known to prolong the QT interval. Electrolyte abnormalities should be corrected before starting treatment, and periodic monitoring of electrolytes and electrocardiograms should be performed during therapy (Falchook et al.).
Contraindications
Dabrafenib is contraindicated in patients with known hypersensitivity to the active substance or any of the excipients used in the formulation. Patients who have experienced severe hypersensitivity reactions, such as anaphylaxis or Stevens-Johnson syndrome, to any BRAF inhibitor should not receive dabrafenib.
The concomitant use of dabrafenib with strong CYP3A4 inducers, such as rifampicin, phenytoin, carbamazepine, or St. John’s wort, is contraindicated due to the potential for decreased plasma concentrations and reduced efficacy of dabrafenib. Alternative therapeutic agents should be considered in these cases (Kainthla et al.).
Dabrafenib is not recommended for use in patients with wild-type BRAF melanoma, as it may promote tumour growth and progression in this population. The use of dabrafenib in combination with other BRAF inhibitors or MEK inhibitors, other than trametinib, is also contraindicated due to the increased risk of adverse events and the lack of established safety and efficacy data for such combinations.
Interactions
The main enzymes responsible for metabolising dabrafenib are cytochrome P450 (CYP) 2C8 and CYP3A4. When dabrafenib is administered in combination with potent inducers or inhibitors of these enzymes, the plasma concentrations of dabrafenib may be markedly changed, which might result in decreased effectiveness or increased toxicity. When feasible, substitute medications should be taken into consideration instead of strong CYP3A4 inhibitors such ketoconazole, itraconazole, clarithromycin, or ritonavir, which should be avoided or used with caution. If concurrent usage cannot be avoided, dosage modifications and careful observation for side effects may be required (Menzies et al.).
It has been demonstrated that dabrafenib induces the CYP3A4 and CYP2C9 enzymes, which may lower the plasma concentrations of substances metabolised by these enzymes, such as digoxin, warfarin, or hormonal contraceptives. When used with dabrafenib, the efficacy of hormonal contraceptives may be decreased; therefore, additional or substitute forms of contraception should be utilised. According to Falchook et al. (“Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial”), patients on medications with a narrow therapeutic index, such as warfarin or digoxin, should have their plasma levels and therapeutic effects closely monitored and their doses adjusted accordingly.
Overdose
In clinical trials, doses of dabrafenib up to 300 mg twice daily have been evaluated, with no apparent increase in toxicity compared to the recommended dose of 150 mg twice daily. However, in the event of a suspected overdose, patients should receive supportive care and be closely monitored for signs and symptoms of adverse reactions. There is no specific antidote for dabrafenib overdose, and treatment should be based on the patient’s clinical presentation and the severity of the overdose.
In cases of severe toxicity, such as life-threatening pyrexia, severe cutaneous adverse reactions, or significant QT prolongation, dabrafenib should be temporarily or permanently discontinued, and appropriate medical interventions should be initiated. Haemodialysis is not expected to enhance the elimination of dabrafenib, as it is highly protein-bound and extensively metabolised (Kainthla et al.).
Patients should be advised to strictly adhere to the prescribed dosage regimen and to report any signs or symptoms of overdose to their healthcare provider immediately. In the event of a missed dose, patients should take the missed dose as soon as they remember, unless it is less than 6 hours before the next scheduled dose, in which case the missed dose should be skipped, and the regular dosing schedule should be resumed.
Additional important information
Development of resilience
The development of dabrafenib as a targeted therapy for BRAF V600E-mutated melanoma represents a significant advancement in the treatment of this aggressive form of cancer. The identification of the BRAF V600E mutation as a driver of melanoma growth and progression has led to the development of selective BRAF inhibitors, such as dabrafenib, which have demonstrated remarkable efficacy in clinical trials. However, the emergence of acquired resistance to BRAF inhibitors has posed a significant challenge in the long-term management of melanoma patients. Combination therapy with MEK inhibitors, such as trametinib, has been shown to delay the onset of resistance and improve patient outcomes (Menzies et al.).
Preclinical and Clinical Studies
Preclinical studies have demonstrated the potent and selective inhibition of BRAF V600E by dabrafenib, leading to the suppression of tumor growth in melanoma xenograft models. In a phase 1 dose-escalation trial, dabrafenib exhibited promising antitumor activity in patients with BRAF V600E-mutated melanoma, including those with untreated brain metastases (Falchook et al.). Subsequent phase 2 and phase 3 trials have confirmed the efficacy of dabrafenib in the treatment of advanced melanoma, with significant improvements in progression-free survival and overall survival compared to standard chemotherapy.
Post-approval studies, Pharmacovigilance and Pharmacokinetic characteristics
Dabrafenib has been approved by regulatory bodies, and since then, a number of post-approval studies have been carried out to assess the drug’s safety and effectiveness in actual clinical settings. These trials have yielded important insights into the treatment of adverse events and resistance development, as well as the long-term results of patients treated with dabrafenib. Efforts have been made to monitor and report any new safety signals related to the use of dabrafenib through pharmacovigilance.
After oral administration, dabrafenib is rapidly absorbed, with a median time to peak plasma concentration of around two hours, according to comprehensive research on its pharmacokinetic properties. According to Kainthla et al., dabrafenib has linear pharmacokinetics spanning a dosage range of 12 to 300 mg and a mean terminal half-life of around 8 hours. Since CYP2C8 and CYP3A4 enzymes are mostly responsible for dabrafenib’s metabolism, there may be drug-drug interactions with significant inducers or inhibitors of these enzymes.
The goal of ongoing research is to find biomarkers that indicate how well a patient will respond to dabrafenib treatment and to investigate new combination approaches for overcoming acquired resistance. The pursuit of better outcomes for patients with metastatic melanoma includes ongoing research into the development of next-generation BRAF inhibitors and the targeting of alternative signalling pathways.
Comparative effectiveness
The comparative effectiveness of dabrafenib has been evaluated in relation to other targeted therapies and standard chemotherapy regimens for the treatment of advanced melanoma. In a phase 3 trial, dabrafenib demonstrated superior progression-free survival and overall survival compared to dacarbazine, a commonly used chemotherapy agent (Hauschild et al.). Furthermore, the combination of dabrafenib and trametinib has shown improved efficacy compared to dabrafenib monotherapy, highlighting the potential benefits of dual inhibition of the MAPK pathway (Long et al.).
Indirect comparisons and network meta-analyses have also been conducted to assess the relative effectiveness of dabrafenib compared to other BRAF and MEK inhibitors. These analyses have suggested that dabrafenib and trametinib combination therapy may have similar efficacy to other BRAF and MEK inhibitor combinations, such as vemurafenib plus cobimetinib or encorafenib plus binimetinib (Devji et al.). However, direct head-to-head comparisons in randomized controlled trials are needed to confirm these findings.
Systematic reviews and meta-analyses
Several systematic reviews and meta-analyses have been conducted to evaluate the efficacy and safety of dabrafenib and other targeted therapies in the treatment of advanced melanoma. A meta-analysis by Xie et al. found that dabrafenib significantly improved progression-free survival and overall survival compared to chemotherapy, with a manageable safety profile. Another meta-analysis by Franken et al. compared the efficacy and safety of BRAF and MEK inhibitors, demonstrating the superiority of combination therapy over monotherapy in terms of response rates, progression-free survival, and overall survival.
These systematic reviews and meta-analyses provide a comprehensive assessment of the available evidence on dabrafenib and other targeted therapies, helping to inform clinical decision-making and guideline development. However, the authors of these studies have also highlighted the limitations of the available data, such as the lack of long-term follow-up and the potential for bias in some of the included trials.
Current research directions and future perspectives
Targeted treatment research for advanced melanoma is now concentrated on a few important areas. Finding predictive biomarkers that can be used to identify people most likely to benefit from dabrafenib and other targeted medicines is an important focus. Research is also being done on the creation of innovative combination methods, such as immunotherapy combined with BRAF and MEK inhibitors (Ascierto et al.).
The creation of methods for overcoming acquired resistance to targeted medicines is a significant area of study. Next-generation BRAF inhibitors and the targeting of other molecular targets have been made possible by the identification of resistance mechanisms, such as secondary BRAF mutations or the activation of alternate signalling pathways.
Potential applications of dabrafenib and other targeted therapy in the early stages of the disease, such as as an adjuvant following surgical resection of high-risk melanoma, provide new prospects for managing advanced melanoma. Research is also being conducted on the combination of targeted therapy with other treatment methods, such as radiation therapy or surgery (Menzies et al.).
The role of dabrafenib and other targeted therapy is expected to continue to grow and improve as our knowledge of the molecular landscape of melanoma continues to advance. The future of personalised treatment for patients with advanced melanoma will be shaped in large part by ongoing clinical trials and translational research endeavours.
Briefly
Dabrafenib is an oral medication used to treat certain types of cancer, particularly melanoma that has spread to other parts of the body or cannot be removed by surgery. As a selective inhibitor of the BRAF V600E mutant kinase, dabrafenib works by blocking the activity of the mutated B-Raf protein, thereby slowing or stopping the growth of cancer cells. Common side effects associated with dabrafenib include fatigue, headache, nausea, vomiting, diarrhoea, constipation, hair loss, and skin-related issues such as rash, dry skin, itching, and redness. Rare but potentially serious side effects may include the development of new primary cutaneous malignancies, severe febrile reactions, hyperglycemia, uveitis, and hemolytic anemia. Precautions and contraindications should be carefully considered before initiating treatment with dabrafenib, and patients should be monitored closely for adverse reactions and potential drug interactions. Ongoing research efforts aim to optimize the use of dabrafenib in combination with other targeted therapies and immunotherapies, as well as to identify predictive biomarkers and strategies to overcome acquired resistance.
ATTENTION: It is of vital importance to never take any medication without the supervision and guidance of a specialised doctor. Consult the package insert of each prescribed medicinal product, as each pharmaceutical company accurately describes the specific specifications for the product, which may undergo regular updates. Note that the trade names mentioned in this article correspond to well-known medicinal products that contain the active substances under analysis. However, there may be variations depending on the composition of each drug. This article focuses on the active substance analysis rather than the drug’s trade name. The reference to trade names is made exclusively for the convenience of readers, who should carefully study the instruction leaflet for each commercial preparation they use. It is necessary to have close cooperation with your attending physician and your pharmacist. The self-administration of any medication carries serious health risks and should be strictly avoided.
Bibliography
- Kainthla, R., Kim, K. B., & Falchook, G. S. “Dabrafenib and its potential for the treatment of metastatic melanoma.” Small Molecules in Oncology, 2014. Springer
- Menzies, A. M., Long, G. V., & Murali, R. “Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial.” Drug design, development and …, 2012. Taylor & Francis
- Falchook, G. S., Long, G. V., Kurzrock, R., Kim, K. B., et al. The Lancet, 2012. The Lancet