Table of Contents
Toggle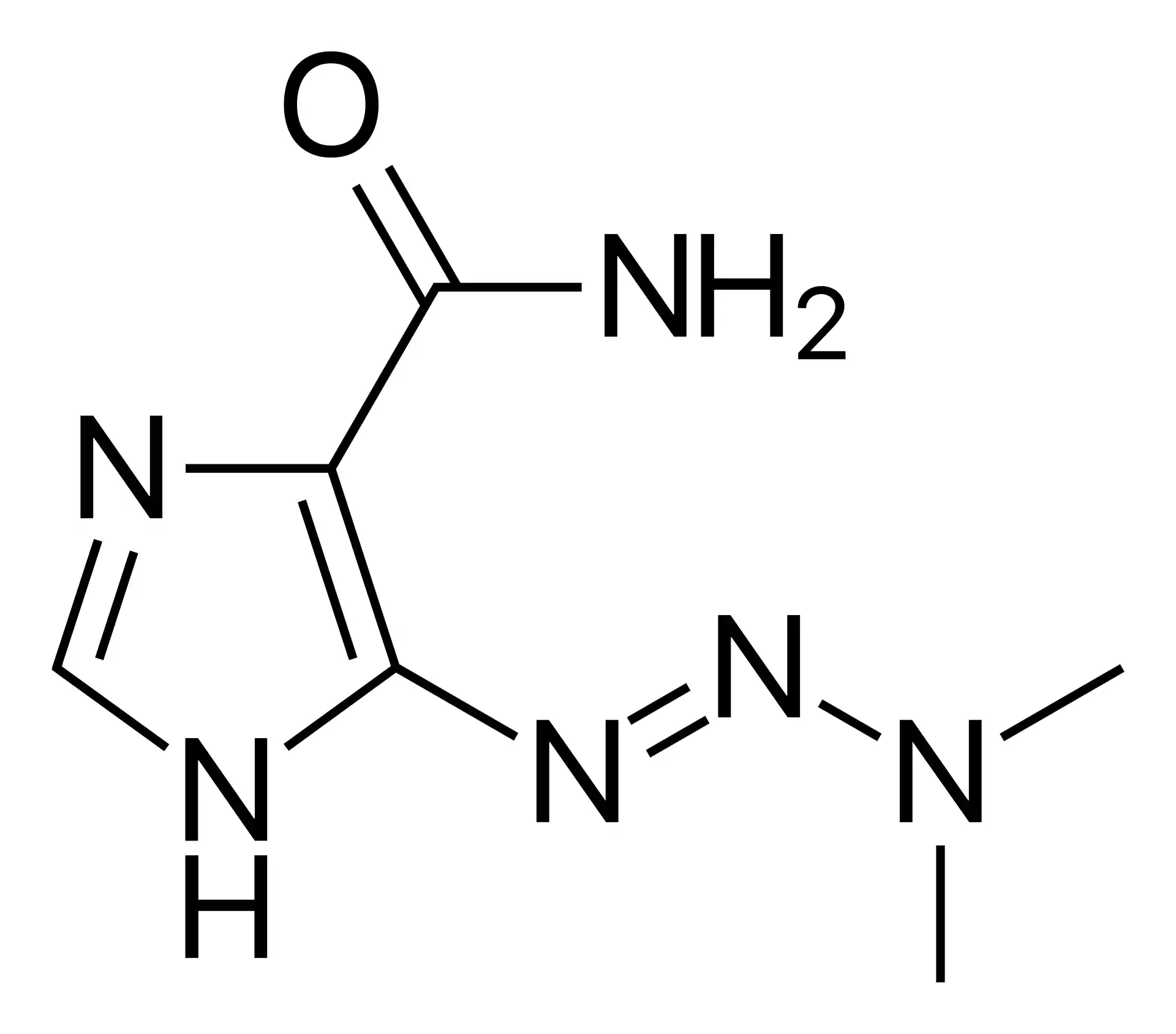
Brand Names: Various around the world
What is Dacarbazine?
Dacarbazine is a chemotherapeutic agent used in the treatment of various cancers, particularly metastatic melanoma. Dacarbazine side effects and uses will be extensively analysed later in this text. Some popular brand names containing this generic medicine include DTIC-Dome, Deticene, Dacarbazine Lipomed, and Dacin. Dacarbazine was first synthesized in the 1960s and has been used as a cancer treatment since the 1970s. In this article, we will analyse several medical research papers and journals concerning the specific active ingredient dacarbazine, rather than focusing on brand names. These sources include works by AA Al-Badr and MM Alodhaib, AMM Eggermont and JM Kirkwood, VC Sileni et al., C Robert et al., and F Teimouri et al., which provide valuable insights into the efficacy and side effects of dacarbazine in cancer treatment.
Chemical Structure and Mechanism of Action
Dacarbazine, also known as imidazole carboxamide or DTIC, is a triazene derivative with the chemical formula C6H10N6O. It is a prodrug that undergoes metabolic activation in the liver, where it is converted to its active form, diazomethane, by the cytochrome P450 enzyme system (Al-Badr and Alodhaib, “Profiles of Drug Substances, Excipients and …”). Diazomethane is a highly reactive alkylating agent that interferes with DNA synthesis and function, leading to cell death. Dacarbazine’s cytotoxic effects are primarily due to its ability to alkylate and crosslink DNA strands, thereby disrupting DNA replication and transcription processes in rapidly dividing cancer cells (Eggermont and Kirkwood, “Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years?”). Additionally, dacarbazine has been shown to inhibit DNA, RNA, and protein synthesis, further contributing to its antitumor activity (Sileni et al., “Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients”). The drug’s selectivity for cancer cells is attributed to their higher rate of proliferation compared to normal cells, making them more susceptible to the effects of alkylating agents like dacarbazine.
Uses
Dacarbazine is primarily indicated for the treatment of metastatic melanoma, a type of skin cancer that has spread to other parts of the body. It has been a standard of care for this condition since the 1970s, despite its limited efficacy as a single agent (Eggermont and Kirkwood). In a phase II randomised study, Sileni et al. compared dacarbazine alone to a combination of dacarbazine, carmustine, cisplatin, and tamoxifen in advanced melanoma patients. The results showed no significant difference in response rates or survival between the two groups, highlighting the need for more effective therapies.
In addition to melanoma, dacarbazine has been used to treat other cancers, such as:
- Hodgkin lymphoma: Dacarbazine is often combined with other chemotherapeutic agents, such as doxorubicin, bleomycin, and vinblastine, in the ABVD regimen for the treatment of Hodgkin lymphoma.
- Soft tissue sarcomas: Dacarbazine has shown activity against various types of soft tissue sarcomas, including leiomyosarcoma, fibrosarcoma, and rhabdomyosarcoma.
- Neuroblastoma: In combination with other chemotherapeutic agents, dacarbazine has been used to treat high-risk neuroblastoma in children.
- Islet cell carcinoma: Dacarbazine has demonstrated some efficacy in the treatment of advanced islet cell carcinoma of the pancreas.
Despite its use in these cancers, dacarbazine’s effectiveness is limited, and it is often combined with other therapies to enhance its antitumor activity. In recent years, newer targeted therapies and immunotherapies have emerged as more promising treatment options for many of these malignancies. For example, in a phase III trial, Robert et al. found that the combination of ipilimumab, an immune checkpoint inhibitor, and dacarbazine significantly improved overall survival compared to dacarbazine alone in previously untreated patients with metastatic melanoma.
The recommended dosage of dacarbazine varies depending on the specific cancer type and treatment protocol. In metastatic melanoma, a typical dose is 200-250 mg/m2 administered intravenously every 21 days. Dose modifications may be necessary based on individual patient tolerability and response to treatment.
While dacarbazine remains a treatment option for certain cancers, its role has diminished in recent years due to the development of more targeted and effective therapies. Ongoing research continues to explore novel combinations and strategies to improve the outcomes of patients with these challenging malignancies.
Dacarbazine Side Effects
Dacarbazine, like many chemotherapeutic agents, can cause a range of side effects due to its non-selective cytotoxic effects on both cancerous and healthy cells. The severity and frequency of these side effects may vary among individuals and can be influenced by factors such as dosage, duration of treatment, and overall health status. It is crucial for patients to discuss potential side effects with their healthcare provider and report any concerns that arise during treatment.
Common Dacarbazine Side Effects
The most common side effects associated with dacarbazine include:
- Nausea and vomiting: These symptoms often occur within 1-3 hours of drug administration and can be managed with antiemetic medications.
- Fatigue and weakness: Many patients experience a general sense of tiredness and lack of energy during treatment.
- Loss of appetite: Dacarbazine may cause a decrease in appetite, leading to unintentional weight loss.
- Hair loss (alopecia): Temporary hair loss is a common side effect of many chemotherapeutic agents, including dacarbazine.
- Skin reactions: Patients may experience rash, itching, or increased sensitivity to sunlight (photosensitivity).
Rare but Possible Dacarbazine Side Effects
Some less common but potentially significant side effects of dacarbazine include:
- Peripheral neuropathy: In rare cases, patients may develop numbness, tingling, or burning sensations in the hands and feet.
- Liver function abnormalities: Dacarbazine can cause elevated liver enzymes, which may require close monitoring and dose adjustments.
- Flu-like symptoms: Patients may experience fever, chills, body aches, and general malaise, particularly in the days following treatment.
- Allergic reactions: In rare instances, patients may develop hypersensitivity reactions to dacarbazine, manifesting as rash, itching, or difficulty breathing.
Serious Dacarbazine Side Effects
Although uncommon, some serious side effects can occur with dacarbazine treatment, including:
- Myelosuppression: Dacarbazine can suppress the production of blood cells in the bone marrow, leading to neutropenia (low white blood cell count), anaemia (low red blood cell count), and thrombocytopenia (low platelet count). This can increase the risk of infections, fatigue, and bleeding (Teimouri et al.).
- Secondary malignancies: Long-term use of alkylating agents like dacarbazine has been associated with an increased risk of developing secondary cancers, such as leukemia or myelodysplastic syndrome.
- infertility: Dacarbazine may cause temporary or permanent infertility in both men and women. Patients should discuss fertility preservation options with their healthcare provider before initiating treatment.
- Pulmonary toxicity: In rare cases, dacarbazine can cause interstitial lung disease or pneumonitis, characterized by cough, dyspnoea, and lung infiltrates (Al-Badr and Alodhaib, “Profiles of Drug Substances, Excipients and …”).
Patients should be closely monitored for any signs of serious side effects during dacarbazine treatment. Prompt reporting of concerning symptoms to a healthcare professional is essential for timely management and prevention of complications.
Warnings
Patients should be informed of a few crucial cautions and warnings before starting dacarbazine medication. These factors are essential for guaranteeing the drug is used safely and effectively while lowering the possibility of negative side effects.
Firstly, dacarbazine should not be administered to patients who have previously experienced hypersensitivity responses to the medication or any of its ingredients. Patients who already have liver or renal disease should also proceed with caution as dacarbazine is mostly metabolised by the liver and eliminated by the kidneys. For certain patients, dose modifications or alternate therapy could be required.
Any past or present medical issues, such as diabetes, autoimmune diseases, or cardiovascular disease, should be disclosed to the patient’s healthcare practitioner since these might affect dacarbazine treatment’s effectiveness and safety. Furthermore, dacarbazine usage may exacerbate immunosuppression that already exists, raising the risk of infections (Eggermont and Kirkwood).
Because dacarbazine might damage foetuses, women who are or will become pregnant should use effective contraception throughout and for many months after starting therapy. Patients should talk to their doctor about dacarbazine’s possible effects on fertility as the drug can result in either temporary or permanent infertility in both men and women.
Dacarbazine users need to be constantly watched for myelosuppressive symptoms as fever, chills, or unusual bleeding or bruises. To evaluate blood cell counts and quickly identify any anomalies, routine blood tests will be carried out. Treatment may be postponed or stopped until blood cell counts improve if significant myelosuppression develops (Robert et al., “Ipilimumab plus dacarbazine for previously untreated metastatic melanoma”).
Dacarbazine may result in photosensitivity, which increases the skin’s vulnerability to sunburn and other sun-related problems. Patients should use sunscreen, wear protective clothes, and limit their time outside during the hours of most sunlight to prevent excessive sun exposure to their skin.
Additionally, patients need to be informed about the possibility of developing secondary cancers while using alkylating medications like dacarbazine over an extended period of time. For the early diagnosis and treatment of any potential secondary malignancies, routine follow-up visits and cancer tests are crucial.
Finally, since dacarbazine might weaken the immune system and raise the risk of vaccine-related problems, patients shouldn’t receive live vaccinations while taking the medication. It is possible to deliver inactivated vaccinations, but the immunosuppressive effects of the medicine may lessen their efficacy.
Patients can avoid the risks related to dacarbazine medication and maximise their overall care plan by carefully collaborating with their healthcare team and being aware of these cautions and warnings.
Precautions
To guarantee patient safety and the best possible therapeutic results while contemplating dacarbazine treatment, a number of measures need to be followed. A complete medical history and physical examination should be performed prior to starting therapy, with special emphasis given to any pre-existing diseases that may affect treatment choices. Since dacarbazine is mostly metabolised by the liver and eliminated by the kidneys, patients with poor liver or kidney function may need dosage changes or alternative therapy (Al-Badr and Alodhaib, “Profiles of Drug Substances, Excipients, and…”).
Because dacarbazine can induce myelosuppression, patients should have regular blood tests to evaluate haematological parameters such white blood cell count, haemoglobin, and platelets. Treatment may need to be postponed or stopped until blood cell counts recover if severe myelosuppression develops. Furthermore, because dacarbazine’s immunosuppressive effects increase the risk of infectious complications, patients should be advised to report any infection-related symptoms, such as fever, chills, or a persistent cough (Teimouri et al., “Efficacy and side effects of dacarbazine in comparison with temozolomide in the treatment of malignant melanoma: a meta-analysis consisting of 1314 patients”).
Because dacarbazine might damage foetuses, women who are planning a pregnancy should use effective contraception throughout and for many months after starting therapy. Before beginning therapy, patients should also talk with their healthcare practitioner about alternatives for fertility preservation and the possible effects of dacarbazine on fertility.
Contraindications
Patients who have a history of known hypersensitivity to dacarbazine or any of its ingredients should not use this medication. When administering dacarbazine, patients who have previously experienced severe allergic responses to chemotherapy drugs should be thoroughly watched for any indications of hypersensitivity.
Dacarbazine shouldn’t be administered to patients who have considerably compromised liver or renal function as it may cause major changes in the drug’s metabolism and excretion, which might enhance its toxicity. For these people, other therapeutic alternatives must to be taken into account (Sileni et al.).
Additionally, because dacarbazine may damage the foetus, it should not be administered to pregnant women. Before beginning treatment, women who are potentially pregnant should get tested, and they should use reliable contraception both during and for several months following medication. Dacarbazine should be stopped right once if a patient becomes pregnant while taking it, and they should be made aware of the possible dangers to the developing baby.
Dacarbazine should not be administered to patients who are actively infected or who are severely immunosuppressed until these symptoms have cleared up since the medication can worsen immune function and raise the risk of infectious consequences (Eggermont and Kirkwood).
Interactions
Dacarbazine may interact with a number of drugs, changing their effectiveness or raising the possibility of negative side effects. Before beginning dacarbazine treatment, patients should disclose to their healthcare practitioner any prescription, over-the-counter, and herbal drugs they are taking.
Levodopa, a medication used to treat Parkinson’s disease, is one such interaction that is noteworthy. Dacarbazine may intensify levodopa’s effects, causing more adverse symptoms such dyskinesia and hallucinations. Levodopa dosage modifications can be required when dacarbazine (Al-Badr and Alodhaib) is used concurrently.
Dacarbazine may potentially have interactions with tamoxifen, carmustine, and cisplatin, among other chemotherapy drugs. Combining these medications might enhance their toxicity since they can intensify each other’s effects (Sileni et al., “Phase II randomised study of dacarbazine alone versus dacarbazine, carmustine, cisplatin, and tamoxifen in advanced melanoma patients”). It is important to closely monitor patients undergoing combination treatment in order to rapidly identify and address any adverse effects.
Furthermore, dacarbazine may interact with immunosuppressive drugs like cyclosporine and corticosteroids, weakening the immune system and raising the risk of infections. When using these drugs in addition to dacarbazine, caution should be used, and patients should be constantly watched for indications of immunosuppression (Robert et al.).
Overdose
Dacarbazine overdose can lead to severe and potentially life-threatening complications. The most common symptoms of overdose include severe nausea, vomiting, diarrhoea, and abdominal pain. In addition, patients may experience excessive myelosuppression, leading to an increased risk of infections, anaemia, and bleeding (Eggermont and Kirkwood, “Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years?”).
In the event of a suspected overdose, immediate medical attention is required. Treatment is primarily supportive and may include:
- Gastric lavage or activated charcoal administration to reduce drug absorption
- Intravenous fluids to maintain hydration and electrolyte balance
- Blood transfusions to manage severe anaemia or thrombocytopenia
- Antibiotics to prevent or treat infections resulting from neutropenia
- Dialysis to remove excess drug from the bloodstream, although the effectiveness of this approach is limited due to dacarbazine’s extensive tissue distribution
Patients who have experienced a dacarbazine overdose should be closely monitored in a hospital setting for several days to detect and manage any complications that may arise. Long-term follow-up may also be necessary to assess the potential impact of the overdose on the patient’s overall health and cancer treatment plan (Teimouri et al.).
Additional Important Information
Development of Resistance
One of the major challenges in the use of dacarbazine for cancer treatment is the development of drug resistance. Tumour cells can acquire resistance to dacarbazine through various mechanisms, such as increased DNA repair, enhanced drug efflux, or alterations in apoptotic pathways (Al-Badr and Alodhaib). The development of resistance can lead to treatment failure and progression of the disease.
To overcome resistance, researchers have investigated the use of combination therapies that target multiple pathways simultaneously. For example, the combination of dacarbazine with immunotherapeutic agents, such as ipilimumab, has shown promising results in improving overall survival in patients with metastatic melanoma (Robert et al., “Ipilimumab plus dacarbazine for previously untreated metastatic melanoma”). Further research is needed to identify novel strategies to prevent or overcome dacarbazine resistance and improve patient outcomes.
Preclinical and Clinical Studies
Preclinical studies have played a crucial role in understanding the mechanisms of action, efficacy, and safety of dacarbazine. The drug’s cytotoxic effects on several cancer cell lines and tumour xenografts have been proven in vitro and in vivo models (Eggermont and Kirkwood). These investigations have also shed light on dacarbazine’s pharmacokinetic and pharmacodynamic characteristics, which has helped determine the best possible dosage schedules.
Clinical trials have assessed dacarbazine’s safety and effectiveness in a variety of cancer forms, most notably melanoma. Phase II and III trials (Sileni et al.) compared combination treatments, such as dacarbazine with carmustine, cisplatin, and tamoxifen, with dacarbazine monotherapy. Combination treatments have occasionally increased response rates, but they haven’t constantly outperformed dacarbazine alone in terms of overall survival.
Clinical trials have lately concentrated on dacarbazine combinations with innovative targeted treatments and immunotherapies. Regulatory regulators approved the combination of dacarbazine and ipilimumab for treating metastatic melanoma in individuals who had not had any prior treatment, based on the groundbreaking phase III trial by Robert et al.
Post-Approval Studies, Pharmacovigilance, and Pharmacokinetic Characteristics
Following the approval of dacarbazine for clinical use, post-approval studies and pharmacovigilance efforts have been ongoing to monitor the drug’s safety and efficacy in real-world settings. These studies help identify rare adverse events and long-term effects that may not have been detected in clinical trials (Teimouri et al., “Efficacy and side effects of dacarbazine in comparison with temozolomide in the treatment of malignant melanoma: a meta-analysis consisting of 1314 patients”).
Pharmacokinetic studies have provided valuable information on the absorption, distribution, metabolism, and excretion of dacarbazine. The drug is administered intravenously and undergoes rapid and extensive metabolism in the liver, primarily by the cytochrome P450 system (Al-Badr and Alodhaib). The active metabolite, MTIC, is formed via demethylation and has a short half-life, necessitating frequent dosing.
Dacarbazine exhibits wide interpatient variability in its pharmacokinetics, which may contribute to differences in efficacy and toxicity among patients. Factors such as age, gender, liver function, and genetic polymorphisms in drug-metabolizing enzymes can influence the pharmacokinetics of dacarbazine (Eggermont and Kirkwood). Understanding these variables is crucial for optimizing dosing strategies and minimizing adverse effects.
Comparative Effectiveness
Dacarbazine has been a standard treatment for metastatic melanoma for several decades, but its effectiveness compared to newer therapies has been a topic of ongoing research. Studies have compared dacarbazine monotherapy with combination regimens and novel targeted therapies or immunotherapies.
A phase II randomised study by Sileni et al. compared dacarbazine alone with a combination of dacarbazine, carmustine, cisplatin, and tamoxifen in patients with advanced melanoma. The results showed no significant difference in response rates or overall survival between the two groups, suggesting that the addition of other chemotherapeutic agents to dacarbazine may not provide substantial benefits.
In contrast, the phase III trial by Robert et al. demonstrated that the combination of ipilimumab, an immune checkpoint inhibitor, with dacarbazine significantly improved overall survival compared to dacarbazine alone in previously untreated patients with metastatic melanoma. This study highlighted the potential of combining immunotherapies with conventional chemotherapy to enhance treatment outcomes.
Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses have been performed to assess dacarbazine’s safety and effectiveness in relation to other therapies for malignant melanoma. In “Efficacy and side effects of dacarbazine in comparison with temozolomide in the treatment of malignant melanoma,” Teimouri et al. conducted a meta-analysis involving 1,314 participants from various trials. Between dacarbazine and temozolomide, another alkylating drug, there was no discernible difference in response rates or overall survival, according to the data. On the other hand, temozolomide was linked to a safer profile with fewer grade 3–4 adverse events.
A thorough summary of the data about dacarbazine’s use in the treatment of melanoma was given in “Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years?” another systematic review by Eggermont and Kirkwood. The authors came to the conclusion that although dacarbazine is still the gold standard, its effectiveness as a stand-alone treatment is restricted, necessitating the use of combination treatments or innovative drugs to enhance patient outcomes.
The comparative efficacy of dacarbazine is clarified by these systematic reviews and meta-analyses, which also aid in guiding treatment choices in clinical settings.
Current Research Directions and Future Perspectives
Current research efforts aim to identify novel strategies to enhance the efficacy of dacarbazine and overcome resistance mechanisms. One promising approach is the development of nanoformulations of dacarbazine, which can improve drug delivery and reduce systemic toxicity (Al-Badr and Alodhaib, “Profiles of Drug Substances, Excipients and Related Methodology”). Preclinical studies have shown that nanoparticle-based formulations of dacarbazine can enhance its antitumor activity and prolong circulation time.
Another area of active research is the identification of biomarkers that can predict response to dacarbazine and guide patient selection. Genetic and epigenetic factors, such as DNA repair pathway alterations and DNA methylation patterns, have been proposed as potential predictive biomarkers (Eggermont and Kirkwood). Validation of these biomarkers in clinical studies could help personalise dacarbazine therapy and improve patient outcomes.
Combination strategies that pair dacarbazine with novel targeted therapies or immunotherapies are also being explored. The success of ipilimumab plus dacarbazine in improving survival in metastatic melanoma has paved the way for investigating other immunotherapy combinations. Ongoing clinical trials are evaluating dacarbazine in combination with PD-1/PD-L1 inhibitors, BRAF inhibitors, and MEK inhibitors, among others (Robert et al.).
As our understanding of the molecular mechanisms underlying cancer progression and drug resistance evolves, new therapeutic targets and strategies will continue to emerge. Integration of dacarbazine with these novel approaches may help optimise its efficacy and overcome limitations in the treatment of melanoma and other cancers.
Briefly
Dacarbazine is a chemotherapeutic agent primarily used in the treatment of metastatic melanoma and other cancers. It causes cell death by interfering with DNA synthesis and acting as an alkylating agent. Although dacarbazine has been a mainstay therapy for melanoma for many years, its effectiveness as a stand-alone medication is constrained. Improved patient outcomes have been demonstrated by combination therapy with immunotherapies or targeted medicines. Fatigue, myelosuppression, nausea, and vomiting are typical side effects. Patients with liver or renal impairment should be monitored carefully, and blood counts should be checked on a regular basis. Research is still being conducted to find ways to combat and improve dacarbazine resistance, which can arise from a variety of processes. Comparing dacarbazine with other therapies has been done using systematic reviews and meta-analyses, which have shed light on the drug’s relative efficacy. Prospects for the future encompass the creation of nanoformulations, the detection of prognostic biomarkers, and the investigation of innovative combination tactics to maximise dacarbazine therapy in the treatment of cancer.
ATTENTION: It is of vital importance to never take any medication without the supervision and guidance of a specialised doctor. Consult the package insert of each prescribed medicinal product, as each pharmaceutical company accurately describes the specific specifications for the product, which may undergo regular updates. Note that the trade names mentioned in this article correspond to well-known medicinal products that contain the active substances under analysis. However, there may be variations depending on the composition of each drug. This article focuses on the active substance analysis rather than the drug’s trade name. The reference to trade names is made exclusively for the convenience of readers, who should carefully study the instruction leaflet for each commercial preparation they use. It is necessary to have close cooperation with your attending physician and your pharmacist. The self-administration of any medication carries serious health risks and should be strictly avoided.
Bibliography
- AA Al-Badr, MM Alodhaib – Profiles of Drug Substances, Excipients and …, 2016 – Elsevier sciencedirect
- AMM Eggermont, JM Kirkwood – European journal of cancer, 2004 – Elsevier sciencedirect
- VC Sileni, R Nortilli, SML Aversa… – Melanoma …, 2001 journals.lww
- C Robert, L Thomas, I Bondarenko… – … England Journal of …, 2011 – Mass Medical Soc nejm.org
- F Teimouri, S Nikfar, M Abdollahi – Melanoma research, 2013 journals.lww


