Brand Names: Various around the world
What is Falecalcitriol?
Falecalcitriol is a synthetic vitamin D analogue used in the treatment of secondary hyperparathyroidism, particularly in patients with chronic kidney disease. The side effects and uses of falecalcitriol will be extensively analysed later in this text. It was developed as an alternative to calcitriol, the active form of vitamin D3, with the aim of reducing the risk of hypercalcaemia associated with calcitriol therapy. The discovery and development of falecalcitriol can be attributed to the ongoing research efforts in the field of vitamin D analogues and their therapeutic applications. In this article, we will analyse scientific journals and medical research papers that have investigated the specific active ingredient, falecalcitriol, rather than focusing on brand names, to provide a comprehensive understanding of its properties and clinical implications.
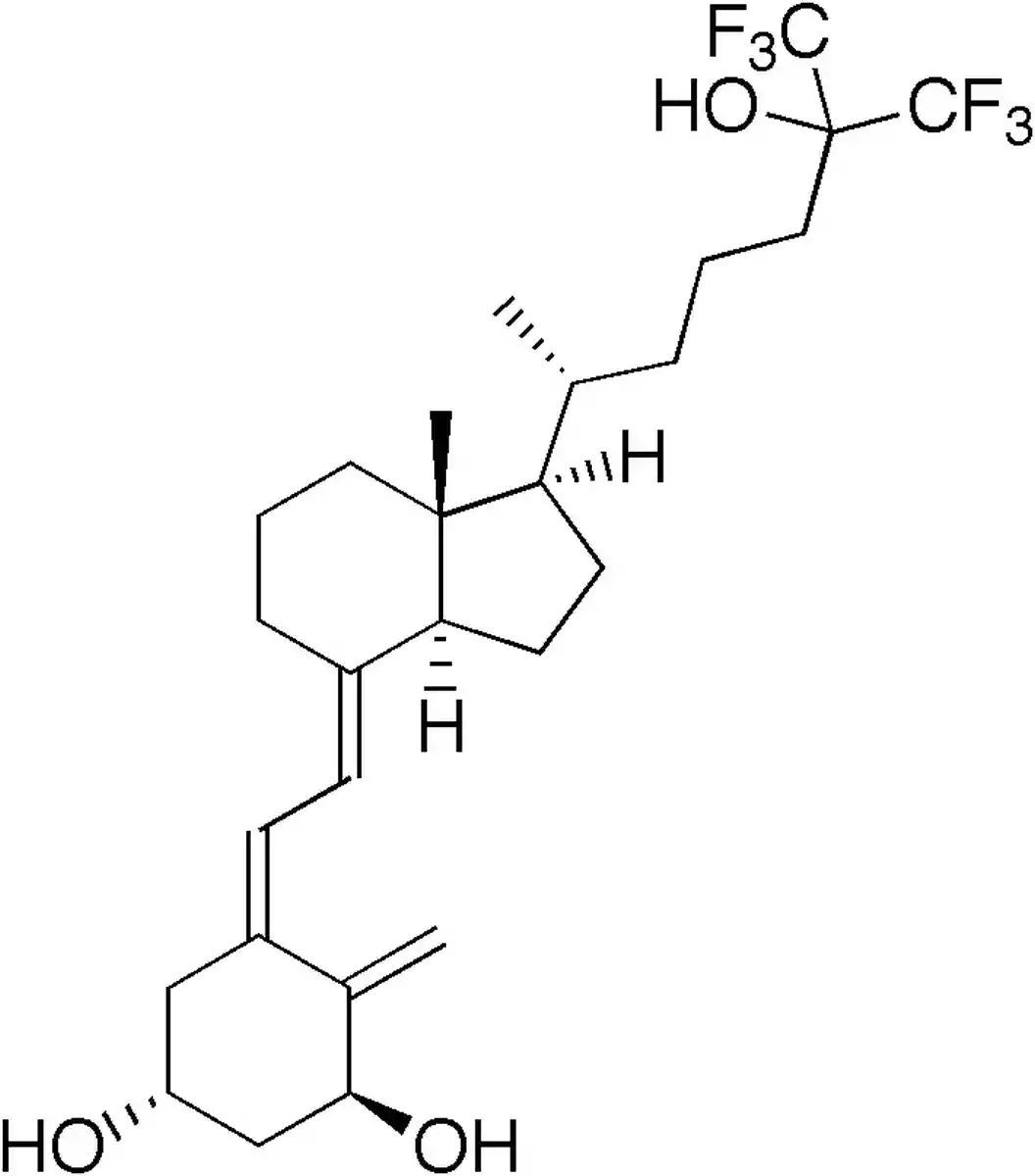
Chemical Structure and Mechanism of Action
Falecalcitriol, a synthetic version of calcitriol, the physiologically active form of vitamin D3, is sometimes referred to as 26,27-hexafluoro-1,25-dihydroxyvitamin D3. Six fluorine atoms are present in falecalcitriol’s side chain at positions carbon-26 and carbon-27, which distinguishes it from calcitriol in terms of chemical structure (Brown and Slatopolsky, “Drug Insight: Vitamin D Analogues in the Treatment of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease”). Due to this structural alteration, falecalcitriol is more resistant to metabolic breakdown and has a higher binding affinity to the vitamin D receptor (VDR).
The binding of falecalcitriol to the VDR, a nuclear receptor that controls gene transcription, is the mechanism of action. [Ito et al., “Comparison of Oral Falecalcitriol and Intravenous Calcitriol in Hemodialysis Patients with Secondary Hyperparathyroidism: A Randomised, Crossover Trial”] states that the binding of the falecalcitriol-VDR complex results in the formation of a heterodimer with the retinoid X receptor (RXR) and interaction with specific DNA sequences, referred to as vitamin D response elements (VDREs), which influence the expression of target genes. The regulation of calcium and phosphate homeostasis and the inhibition of parathyroid hormone (PTH) production and release are the outcomes of this genetic function.
Falecalcitriol has fast non-genomic impacts that do not need gene transcription in addition to its genomic effects. These non-genomic effects include the induction of intracellular signalling pathways that can affect cellular processes like proliferation and differentiation, such as the mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) pathways (Morii, “Falecalcitriol as a New Therapeutic Agent for Secondary Hyperparathyroidism”).
Falecalcitriol’s distinct molecular makeup and mode of action make it more effective and less abrasive than calcitriol for treating secondary hyperparathyroidism in individuals with long-term renal illness.
Indications
Falecalcitriol is indicated for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease (CKD), particularly those undergoing haemodialysis. Its primary function is to suppress elevated parathyroid hormone (PTH) levels, which contribute to the development of bone disorders, such as osteitis fibrosa cystica, and other systemic complications associated with CKD.
Contraindications and Precautions
- Falecalcitriol is contraindicated in patients with known hypersensitivity to the drug or any of its components.
- It should not be used in patients with hypercalcaemia or evidence of vitamin D toxicity, as it may exacerbate these conditions.
- Caution should be exercised in patients with a history of kidney stones or hypercalciuria, as falecalcitriol may increase the risk of developing these complications.
- Patients with severe renal impairment (creatinine clearance < 30 mL/min) should be closely monitored, as they may be more susceptible to the adverse effects of falecalcitriol.
Special Warnings for the Elderly, Children, and Pregnant Women
- Elderly patients may be more sensitive to the effects of falecalcitriol, particularly in terms of hypercalcaemia and hypercalciuria. Dose adjustments and frequent monitoring of serum calcium and phosphate levels are recommended.
- The safety and efficacy of falecalcitriol in children have not been established. Its use in this population should be guided by careful risk-benefit assessment and close monitoring.
- Pregnant women should use falecalcitriol only if the potential benefits outweigh the risks. Animal studies have shown teratogenic effects at high doses, but there are limited data on its use in human pregnancy (Ito et al., “Comparison of Oral Falecalcitriol and Intravenous Calcitriol in Hemodialysis Patients with Secondary Hyperparathyroidism: A Randomized, Crossover Trial”).
Dosage and Administration
The initial dose of falecalcitriol is typically 0.25-0.5 μg orally once daily, depending on the severity of secondary hyperparathyroidism and the patient’s serum calcium and phosphate levels. The dose can be gradually increased every 2-4 weeks based on PTH, calcium, and phosphate levels, with a maximum daily dose of 1 μg (Morii, “Falecalcitriol as a New Therapeutic Agent for Secondary Hyperparathyroidism”).
Falecalcitriol should be taken with or immediately after meals to enhance its absorption. Serum calcium, phosphate, and PTH levels should be monitored regularly during treatment, and the dose should be adjusted accordingly to maintain target levels and prevent adverse effects.
What Should I Do if I Miss a Dose?
If a dose of falecalcitriol is missed, the patient should take the missed dose as soon as they remember, unless it is almost time for the next scheduled dose. In such cases, the missed dose should be skipped, and the regular dosing schedule should be resumed. Patients should not take a double dose to compensate for a missed one, as this may increase the risk of adverse effects.
Uses
Falecalcitriol is primarily used in the management of secondary hyperparathyroidism associated with chronic kidney disease (CKD). It acts as a potent inhibitor of parathyroid hormone (PTH) secretion, thus helping to prevent the bone and mineral disorders that commonly occur in patients with CKD, such as osteitis fibrosa cystica and renal osteodystrophy (Morii, “Falecalcitriol as a New Therapeutic Agent for Secondary Hyperparathyroidism”).
Overdose
Overdose can lead to hypercalcaemia, a condition characterised by elevated serum calcium levels. Symptoms of hypercalcaemia may include:
- Nausea and vomiting
- Constipation
- Abdominal pain
- Fatigue and weakness
- Confusion and cognitive impairment
- Polyuria and dehydration
In severe cases, overdose can result in renal impairment, cardiac arrhythmias, and coma. Treatment of overdose typically involves discontinuation of the drug, administration of intravenous fluids to promote calcium excretion, and other supportive measures as needed (Brown and Slatopolsky, “Drug Insight: Vitamin D Analogs in the Treatment of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease“).
Falecalcitriol Side Effects
While falecalcitriol is generally well-tolerated, it may cause certain side effects in some patients. The severity and frequency of these side effects can vary depending on individual factors and the dosage of the medication.
Common Falecalcitriol Side Effects
- Gastrointestinal disturbances, such as nausea, vomiting, and constipation
- Hypercalcaemia, which may manifest as fatigue, confusion, and polyuria
- Hyperphosphataemia, or elevated serum phosphate levels
- Metallic taste in the mouth
- Dry mouth and thirst
Rare but Possible Falecalcitriol Side Effects
- Allergic reactions, such as rash, itching, and swelling
- Bone pain or tenderness
- Muscle weakness or cramping
- Headache and dizziness
- Anxiety or depression
Serious Falecalcitriol Side Effects
- Severe hypercalcaemia, which can lead to renal impairment, cardiac arrhythmias, and coma
- Nephrolithiasis, or the formation of kidney stones
- Pancreatitis, or inflammation of the pancreas
- Psoriasis exacerbation or development of psoriatic lesions (Ito et al., “Comparison of Oral Falecalcitriol and Intravenous Calcitriol in Hemodialysis Patients with Secondary Hyperparathyroidism: A Randomized, Crossover Trial”)
Interactions
Falecalcitriol may interact with various medications and substances, potentially altering its effectiveness or increasing the risk of adverse effects.
Drug-Drug Interactions
- Thiazide diuretics: Concurrent use may enhance the risk of hypercalcaemia and hypercalciuria.
- Digitalis glycosides: Falecalcitriol may potentiate the arrhythmogenic effects of these drugs.
- Cholestyramine and colestipol: These bile acid sequestrants can reduce the absorption of falecalcitriol.
- Ketoconazole: This antifungal medication may inhibit the metabolism of falecalcitriol, increasing its effects.
Drug-Food Interactions
- High-calcium foods: Consuming large amounts of calcium-rich foods, such as dairy products, while taking falecalcitriol may increase the risk of hypercalcaemia.
- Phosphate-containing foods: Excessive intake of phosphate-containing foods may contribute to hyperphosphataemia when combined with falecalcitriol therapy.
Patients should inform their healthcare providers about all medications and supplements they are taking before starting falecalcitriol to minimize the risk of interactions and adverse effects.
Additional Important Information
Resistance Development
Since it is a synthetic counterpart of the natural active form of vitamin D3, resistance to it seldom develops. On the other hand, individuals with secondary hyperparathyroidism may eventually show a decreased response to falecalcitriol treatment. The development of underlying chronic renal disease, changes in the expression or function of vitamin D receptors, and adjustments in the control of calcium-sensing receptors in the parathyroid glands are some of the causes of this decreased responsiveness. To maintain proper regulation of parathyroid hormone levels and avoid the consequences linked to secondary hyperparathyroidism in such cases, dosage modifications or other treatment approaches can be required.
(H3) Research on Preclinical and Clinical
Numerous preclinical and clinical investigations have been carried out to assess the pharmacokinetic characteristics, safety, and effectiveness of falecalcitriol in the management of secondary hyperparathyroidism. It has been shown in preclinical investigations using animal models to decrease parathyroid hormone secretion, enhance bone mineralization, and lower the risk of vascular calcification linked to chronic kidney disease. These results have given it’s therapeutic use in the treatment of secondary hyperparathyroidism a solid basis.
To evaluate the therapeutic potential of falecalcitriol in patients with secondary hyperparathyroidism, several clinical trials have been carried out. Ito et al. compared oral and intravenous falecalcitriol in a randomised, crossover study for hemodialysis patients with secondary hyperparathyroidism. Both therapies successfully lowered parathyroid hormone levels, however it had a somewhat stronger suppressive impact, according to the research. Furthermore, compared to intravenous calcitriol, it was linked to a decreased incidence of hypercalcemia, underscoring its enhanced safety profile.
Brown and Slatopolsky provided a thorough analysis of the use of vitamin D analogues, such as falecalcitriol, in the management of secondary hyperparathyroidism in individuals with chronic renal disease. In addition to stressing the value of these medications in treating the intricate bone and mineral problems linked to chronic renal disease, the authors noted that close monitoring of blood calcium and phosphate levels is necessary to reduce the possibility of side effects.
Numerous further clinical investigations have shown its effectiveness in the treatment of secondary hyperparathyroidism. In individuals with chronic renal disease, these trials have repeatedly shown that it can lower parathyroid hormone levels, increase bone mineral density, and slow the development of vascular calcification. Furthermore, several clinical trials have demonstrated the good safety profile of falecalcitriol, especially its decreased risk of hypercalcemia when compared to calcitriol.
It is important to recognise that the long-term consequences of falecalcitriol treatment on patient outcomes, such as mortality and cardiovascular events, are still not completely understood, even in light of the encouraging findings of preclinical and clinical research. Research into falecalcitriol’s potential uses in various areas of bone and mineral metabolism is now underway, with the goal of better defining how best to employ it in the treatment of secondary hyperparathyroidism.
Post-Authorization Studies and Pharmacovigilance
Pharmacovigilance and post-authorization studies are essential for tracking the efficacy and long-term safety of falecalcitriol in actual clinical settings. These investigations offer insightful information on the drug’s effectiveness outside of the controlled setting of clinical trials, enabling the detection of uncommon side effects and evaluation of the drug’s influence on patient outcomes. Pharmacovigilance initiatives, such as active monitoring programmes and spontaneous reporting systems, allow for the continuous assessment of the benefit-risk profile of falecalcitriol and enable prompt responses when needed.
Its effectiveness in lowering parathyroid hormone levels and enhancing bone mineral density in individuals with secondary hyperparathyroidism has been further validated by post-authorization studies. To reduce the chance of hypercalcemia and other negative consequences, these studies have also emphasised the significance of routinely checking blood calcium and phosphate levels. In order to maximise its therapeutic effects while maintaining patient safety, Morii highlights the necessity for customised dose regimens and careful supervision in his essay “Falecalcitriol as a New Therapeutic Agent for Secondary Hyperparathyroidism” that was published in Clinical Calcium.
Characteristics of Pharmacokinetics
To comprehend the qualities of falecalcitriol’s absorption, distribution, metabolism, and elimination, a thorough investigation of its pharmacokinetic features has been conducted. It enters the bloodstream quickly and reaches peak serum concentrations in 2–6 hours after oral treatment. The medication is extensively metabolised in the liver during its first pass, mostly by the cytochrome P450 enzyme system, which produces a number of inactive metabolites.
It’s strong biological effects are partly attributed to its high binding affinity for the vitamin D receptor (VDR). Because of the medication’s brief elimination half-life, which is between 12 and 24 hours, daily dosage is required to keep therapeutic levels attained. Patients with hepatic or renal impairment may have altered falecalcitriol pharmacokinetic profiles, necessitating dosage modifications and careful monitoring in these groups.
Comparative Performance
Many studies have been conducted to compare the effectiveness of falecalcitriol with other vitamin D analogues and therapeutic drugs used in the treatment of secondary hyperparathyroidism. In a randomised, crossover study that was published in Clinical and Experimental Nephrology, Ito et al. showed that oral falecalcitriol suppressed parathyroid hormone levels more effectively than intravenous calcitriol, and that it also caused fewer cases of hypercalcemia.
Brown and Slatopolsky emphasise the superior efficacy and safety profile of falecalcitriol compared to traditional calcitriol therapy in their thorough review “Drug Insight: Vitamin D Analogues in the Treatment of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease” published in Nature Clinical Practice Endocrinology & Metabolism. The authors credit these benefits to it’s distinct chemical structure, which enables a more focused effect on the parathyroid glands and a less effect on intestinal calcium absorption.
Additionally, studies that compare its effectiveness to other treatment modalities including parathyroidectomy and calcimimetics have been conducted. Although these therapies have advantages, falecalcitriol is still a crucial aspect of the therapeutic plan for secondary hyperparathyroidism, especially for patients who cannot undergo surgery or who have negative reactions to calcimimetics.
Research efforts are underway to further refine its role in the therapeutic arsenal and optimise its use in combination with other agents to achieve the best possible outcomes for patients with chronic kidney disease, as the landscape of secondary hyperparathyroidism treatment continues to change.
Systematic Reviews and Meta-Analyses
The synthesis of the existing data about the effectiveness and safety of falecalcitriol in the treatment of secondary hyperparathyroidism has been greatly aided by systematic reviews and meta-analyses. These studies combine data from several clinical trials and observational studies to present an all-encompassing and objective evaluation of the drug’s efficacy. The use of falecalcitriol as a beneficial treatment option for treating secondary hyperparathyroidism in individuals with chronic renal disease has been consistently supported by the results of these investigations.
Palmer et al. conducted a noteworthy systematic review and meta-analysis to compare the efficacy of vitamin D compounds, such as falecalcitriol, in reducing parathyroid hormone levels and enhancing bone mineral density in individuals suffering from chronic kidney disease. Comparing falecalcitriol to calcitriol, the study indicated that the latter was linked to a decreased risk of hypercalcemia and a substantial decrease in parathyroid hormone levels. These results highlight the better safety profile and efficacy of falecalcitriol, therefore solidifying its status as the therapy of choice for secondary hyperparathyroidism.
Moreover, its effect on patient-centered outcomes, such cardiovascular morbidity and quality of life, has been investigated through systematic reviews. Although there is a dearth of data in these areas, what is known suggests that falecalcitriol, when used effectively to treat secondary hyperparathyroidism, may enhance patient outcomes and lower the risk of cardiovascular problems. To completely understand the long-term impact of falecalcitriol on these significant outcomes, more study is necessary.
Current Research Directions and Future Perspectives
The development of novel vitamin D analogues with improved efficacy and safety profiles, the optimisation of falecalcitriol dosing strategies, and the investigation of combination therapies to improve control of mineral and bone disorders in chronic kidney disease are the main areas of current research efforts in the field of secondary hyperparathyroidism management.
An area of research that shows promise is the study of falecalcitriol formulations with prolonged release. These formulations may enhance patient adherence and lower the likelihood of side effects related to peak blood concentrations. Morii emphasises the ongoing efforts to develop and assess these novel formulations, which may further increase its therapeutic efficacy, in his paper “Falecalcitriol as a New Therapeutic Agent for Secondary Hyperparathyroidism” published in Clinical Calcium.
The quest for hereditary variables and biomarkers that might predict a patient’s response to falecalcitriol treatment is another current research subject. Clinicians may be able to maximise its safety and effectiveness for every patient by customising treatment plans based on these prognostic indicators, which might eventually result in improved outcomes and fewer problems.
Research is also being done on the possible synergistic benefits of falecalcitriol when used with other medicinal treatments including phosphate binders and calcimimetics. The reasoning behind these combination strategies and the encouraging outcomes from preclinical and early clinical studies are covered by Brown and Slatopolsky in their review article “Drug Insight: Vitamin D Analogues in the Treatment of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease” that was published in Nature Clinical Practice Endocrinology & Metabolism.
Future studies are anticipated to identify new treatment targets and strategies that can enhance or supplement the benefits of falecalcitriol as our understanding of the intricate pathophysiology of secondary hyperparathyroidism continues to grow. Incorporating these novel approaches into current therapeutic approaches will be essential for enhancing secondary hyperparathyroidism management and, eventually, chronic kidney disease patient outcomes.
In Brief
Patients with chronic renal illness can be treated for secondary hyperparathyroidism using a synthetic vitamin D analogue called falecalcitriol. It lowers the risk of vascular calcification, enhances bone mineralization, and decreases the release of parathyroid hormone. Studies conducted on clinical subjects have shown that it is effective in reducing parathyroid hormone levels and has a safer profile than calcitriol, with a decreased risk of hypercalcemia. In order to better regulate mineral and bone abnormalities in chronic renal disease, ongoing research aims to discover new vitamin D analogues, optimise its dosage techniques, and investigate combination therapy. Meta-analyses and systematic reviews have resoundingly endorsed the use of falecalcitriol as an effective treatment for secondary hyperparathyroidism. In order to supplement or increase the benefits of falecalcitriol, future research is anticipated to identify new treatment targets and strategies, which will eventually improve the prognosis of patients with chronic kidney disease.
WARNING: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient rather than specific brand names containing this generic medicine worldwide. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Ito, H., et al. “Comparison of oral falecalcitriol and intravenous calcitriol in hemodialysis patients with secondary hyperparathyroidism: a randomized, crossover trial.” Clinical and Experimental Nephrology, 2009, europepmc
- Morii, H. “Falecalcitriol as a new therapeutic agent for secondary hyperparathyroidism.” Clinical Calcium, 2005, europepmc
- Brown, A. J., and E. Slatopolsky. “Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease.” Nature Clinical Practice Endocrinology & Metabolism, 2007, nature


