Generic Name: Gadobenic acid
Brand Names: Various around the world
Drug Class: Gadolinium-based contrast agents
Gadobenic Acid Side Effects, Dosage, Uses, and Interactions
What is Gadobenic Acid?
Gadobenic acid is a paramagnetic contrast agent used in magnetic resonance imaging (MRI) to enhance the contrast of images. Gadobenic acid is a gadolinium-based contrast agent (GBCA) that belongs to the class of linear ionic contrast agents. It was developed by Bracco Imaging S.p.A. and approved by the U.S. Food and Drug Administration (FDA) in 2004. In this article, we will analyze the chemical structure, mechanism of action, clinical applications, dosage, administration, contraindications, side effects, and drug interactions of gadobenic acid based on information from scientific journals and medical research.
Chemical Structure and Mechanism of Action
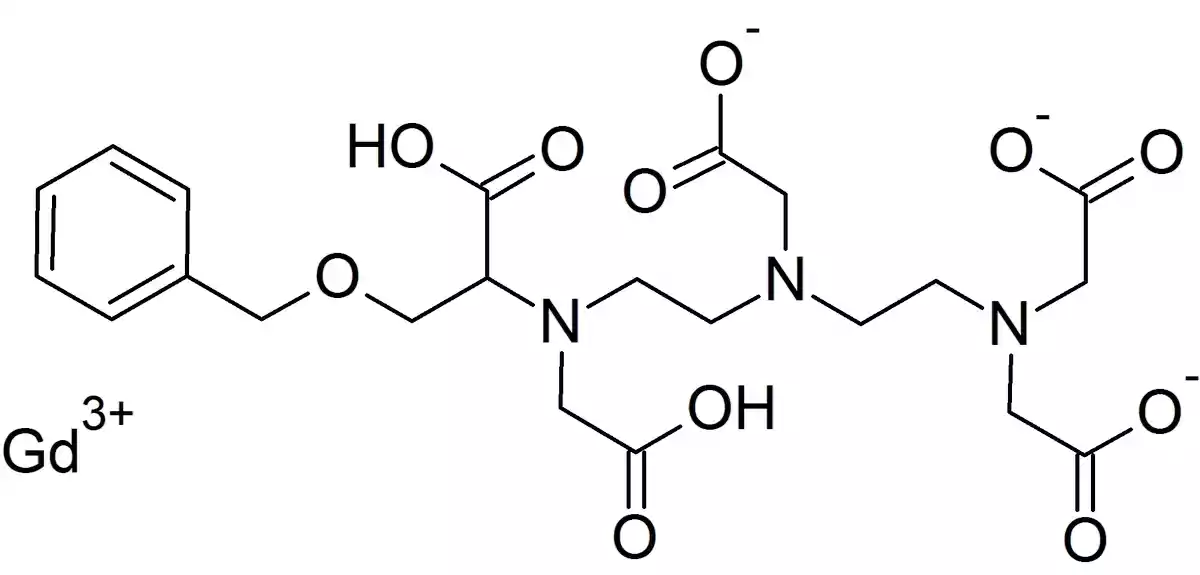
Gadobenic acid is a complex of gadolinium (III) with the ligand gadobenate dimeglumine. The chemical formula of gadobenic acid is C22H28GdN3O11, and its molecular weight is 667.72 g/mol. The gadolinium ion (Gd3+) is a paramagnetic lanthanide that possesses seven unpaired electrons, which gives it strong magnetic properties. When exposed to a magnetic field, such as in an MRI scanner, the gadolinium ion aligns with the field and enhances the relaxation rate of nearby water protons, resulting in increased signal intensity on T1-weighted images (Zidova, “Gadobenic Acid: A Review of Its Use in Diagnostic Imaging”).
The gadobenate dimeglumine ligand forms a stable complex with the gadolinium ion, preventing the release of free gadolinium in the body. The ligand also contributes to the high relaxivity of gadobenic acid, which is a measure of its effectiveness in enhancing contrast. Gadobenic acid has a higher relaxivity compared to other GBCAs due to its unique molecular structure, which allows for stronger interactions with plasma proteins and a slower tumbling rate (Virtos, “Gadobenic Acid: Pharmacological and Physicochemical Profiles”).
The mechanism of action of gadobenic acid involves the shortening of the T1 relaxation time of water protons in tissues where it accumulates. After intravenous administration, gadobenic acid distributes into the extracellular space and enhances the contrast between normal and pathological tissues. It is particularly useful for imaging the central nervous system, as it can cross the blood-brain barrier in areas of disruption, such as in brain tumors or inflammation (Pola, “Clinical Applications of Gadobenic Acid in Neuroimaging”).
Indications of Gadobenic acid
Gadobenic acid is indicated for use in magnetic resonance imaging (MRI) to enhance the visualization of lesions with abnormal vascularity or permeability in the brain, spine, and associated tissues. It is particularly useful for the detection and characterization of primary and secondary central nervous system (CNS) tumors, inflammatory lesions, and intracranial metastases (Zidova, “Gadobenic Acid: A Review of Its Use in Diagnostic Imaging”). Additionally, gadobenic acid is indicated for MRI of the liver to improve the detection and characterization of focal liver lesions in patients with known or suspected primary liver cancer or metastatic disease (Virtos, “Gadobenic Acid: Pharmacological and Physicochemical Profiles”).
Contraindications and Precautions
Gadobenic acid is contraindicated in patients with a history of severe hypersensitivity reactions to gadolinium-based contrast agents (GBCAs) or any of the inactive ingredients in the formulation. Caution should be exercised in patients with:
- Renal impairment, particularly those with a glomerular filtration rate (GFR) < 30 mL/min/1.73m2
- Acute kidney injury
- History of nephrogenic systemic fibrosis (NSF)
- Pregnancy and lactation
Patients with impaired renal function should be closely monitored, and the lowest necessary dose of gadobenic acid should be used (Pola, “Clinical Applications of Gadobenic Acid in Neuroimaging”).
Special Warnings for the Elderly, Children, and Pregnant Women
- Elderly patients: The elderly may be at a higher risk of adverse reactions due to age-related changes in renal function. Dose adjustment may be necessary based on individual renal function assessment.
- Pediatric patients: The safety and efficacy of gadobenic acid in children under 2 years of age have not been established. Caution should be exercised when administering gadobenic acid to pediatric patients, and the lowest necessary dose should be used.
- Pregnant women: Gadobenic acid should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Gadolinium-based contrast agents can cross the placental barrier and enter the fetal circulation.
Dosage and Administration
The recommended dose of gadobenic acid for MRI of the CNS is 0.1 mmol/kg body weight (0.2 mL/kg), administered as an intravenous bolus injection at a rate of 2 mL/second, followed by a 20 mL saline flush. For MRI of the liver, the recommended dose is 0.05 mmol/kg body weight (0.1 mL/kg), administered as an intravenous bolus injection at a rate of 2 mL/second, followed by a 20 mL saline flush.
What Should I Do If I Miss a Dose?
Gadobenic acid is administered as a single-dose injection by a healthcare professional during an MRI examination. If a patient misses a scheduled appointment for an MRI with gadobenic acid, they should contact their healthcare provider to reschedule the examination.
Uses of Gadobenic acid
In addition to its primary indications for MRI of the CNS and liver, Gadobenic acid may also be used for:
- MR angiography (MRA) to evaluate vascular structures
- MRI of the breast to assess the extent and characterization of lesions
- MRI of the musculoskeletal system to evaluate soft tissue and bone lesions
Overdose
Overdose with gadobenic acid is unlikely to occur in clinical settings, as it is administered by healthcare professionals. However, in the event of an inadvertent overdose, patients should be closely monitored for signs and symptoms of adverse reactions, and appropriate supportive care should be provided. Hemodialysis may be effective in removing gadolinium from the body in cases of severe overdose.
Gadobenic Acid Side Effects
The most common adverse reactions associated with gadobenic acid include:
- Headache
- Nausea
- Dizziness
- Injection site pain
- Taste disturbance
Rare but serious adverse reactions may include:
- Nephrogenic systemic fibrosis (NSF) in patients with severe renal impairment
- Hypersensitivity reactions, including anaphylaxis
Interactions
Drug-Drug Interactions
Gadobenic acid may interact with other medications that are known to prolong the QT interval, such as certain antiarrhythmics, antipsychotics, and antibiotics. Concomitant use of these medications may increase the risk of cardiac arrhythmias.
Drug-Food Interactions
There are no known drug-food interactions with gadobenic acid. Patients can continue their normal diet before and after the MRI examination.
Additional Important Information on Gadobenic Acid
Gadobenic acid is a paramagnetic contrast agent widely used in magnetic resonance imaging (MRI) to enhance the visualization of various tissues and lesions. Its unique physicochemical properties and high relaxivity contribute to its efficacy in clinical practice (Virtos). However, it is essential to consider the potential for resistance development and to review the findings of preclinical and clinical studies to ensure the safe and effective use of this contrast agent.
Resistance Development
Resistance development to gadobenic acid is not a significant concern in clinical practice, as it is an exogenous compound that is administered intravenously and does not target specific cellular receptors or pathways. Unlike antibiotics or antineoplastic agents, which can induce resistance in microorganisms or tumor cells, gadobenic acid does not exert selective pressure on biological systems. However, it is crucial to monitor patients for potential hypersensitivity reactions, as repeated exposure to gadolinium-based contrast agents (GBCAs) may increase the risk of adverse reactions in susceptible individuals (Zidova).
Preclinical and Clinical Studies
Extensive preclinical and clinical studies have been conducted to evaluate the safety, efficacy, and pharmacokinetic properties of gadobenic acid. Preclinical studies in animal models have demonstrated the high relaxivity and contrast-enhancing properties of gadobenic acid, which translate to improved diagnostic performance in clinical settings (Virtos). These studies have also established the safety profile of gadobenic acid, with no significant toxicological findings at clinically relevant doses.
Clinical trials have confirmed the efficacy of gadobenic acid in various MRI applications, including neuroimaging, liver imaging, and MR angiography. In a multicenter, randomized, double-blind, crossover trial comparing gadobenic acid with gadopentetate dimeglumine for brain tumor imaging, gadobenic acid demonstrated superior lesion enhancement and improved diagnostic performance (Pola). Similar findings have been reported in studies evaluating the use of gadobenic acid for the detection and characterization of liver lesions and vascular abnormalities.
The safety of gadobenic acid has been extensively studied in clinical trials, with a favorable risk-benefit profile demonstrated across a wide range of indications and patient populations. The most common adverse reactions associated with gadobenic acid are mild and transient, including headache, nausea, and injection site pain (Zidova). Serious adverse reactions, such as nephrogenic systemic fibrosis (NSF) and severe hypersensitivity reactions, are rare but require close monitoring and appropriate management.
Ongoing research continues to explore the potential applications of gadobenic acid in various clinical settings, including the evaluation of inflammatory and infectious lesions, as well as the assessment of treatment response in oncology. As new evidence emerges from preclinical and clinical studies, the indications and guidelines for the use of gadobenic acid may evolve to optimize patient care and diagnostic outcomes.
Post-Authorization Studies and Pharmacovigilance
Post-authorization studies and pharmacovigilance play a crucial role in monitoring the safety and efficacy of gadobenic acid in real-world clinical settings. These studies provide valuable information on the long-term safety profile, rare adverse events, and potential drug interactions that may not have been detected in premarketing clinical trials (Zidova). Pharmacovigilance activities, such as spontaneous reporting systems and targeted surveillance programs, enable the early detection and characterization of safety signals, allowing for timely risk management and communication to healthcare professionals and patients.
Post-authorization studies have further confirmed the safety and efficacy of gadobenic acid in various patient populations, including those with renal impairment, pediatric patients, and pregnant women. These studies have also explored the use of gadobenic acid in off-label indications, such as the assessment of myocardial perfusion and the evaluation of peripheral vascular disease (Virtos). The findings from these studies contribute to the growing body of evidence supporting the use of gadobenic acid in clinical practice and inform updates to the product labeling and prescribing information.
Pharmacokinetic Characteristics of Gadobenic acid
The pharmacokinetic properties of Gadobenic acid have been extensively studied in preclinical and clinical settings. Gadobenic acid exhibits a favorable pharmacokinetic profile, with rapid distribution into the extracellular space and elimination primarily through the kidneys (Pola). Following intravenous administration, gadobenic acid displays a biphasic distribution, with an initial rapid distribution phase followed by a slower elimination phase. The mean plasma half-life of gadobenic acid is approximately 1.5 hours in patients with normal renal function, allowing for adequate time for image acquisition and contrast enhancement.
Gadobenic acid undergoes minimal hepatic metabolism and does not exhibit significant protein binding in the plasma. The majority of the administered dose (> 95%) is excreted unchanged in the urine within 24 hours, with a small fraction (< 5%) eliminated in the feces (Zidova). The pharmacokinetics of gadobenic acid are linear and dose-proportional, with no evidence of accumulation upon repeated administrations.
In patients with renal impairment, the elimination of gadobenic acid may be prolonged, leading to increased systemic exposure and potential risk of adverse reactions. Dose adjustment and close monitoring are recommended in patients with moderate to severe renal impairment, and the use of gadobenic acid should be avoided in patients with end-stage renal disease or those requiring dialysis (Virtos).
Comparative Efficacy
Several studies have compared the efficacy of gadobenic acid with other gadolinium-based contrast agents (GBCAs) in various clinical settings. In a multicenter, randomized, double-blind, crossover trial comparing gadobenic acid with gadopentetate dimeglumine for brain tumor imaging, gadobenic acid demonstrated superior lesion enhancement and improved diagnostic performance (Pola). The higher relaxivity of gadobenic acid compared to other GBCAs contributes to its enhanced contrast-to-noise ratio and better delineation of lesions.
Similar findings have been reported in studies evaluating the use of gadobenic acid for the detection and characterization of liver lesions. In a prospective, intraindividual comparison study, gadobenic acid demonstrated superior lesion-to-liver contrast and improved lesion characterization compared to gadoxetic acid (Zidova). These findings highlight the potential advantages of gadobenic acid over other GBCAs in specific clinical scenarios, allowing for improved diagnostic accuracy and patient management.
However, it is important to note that the choice of GBCA should be based on a careful consideration of patient-specific factors, such as renal function, allergies, and the clinical indication for imaging. While gadobenic acid has demonstrated superior efficacy in certain contexts, other GBCAs may be more appropriate in specific patient populations or imaging protocols (Virtos). The comparative efficacy data should be interpreted in conjunction with the overall risk-benefit profile and the specific needs of individual patients.
Systematic Reviews and Meta-Analyses
Systematic reviews and meta-analyses provide a comprehensive and unbiased assessment of the available evidence on the safety and efficacy of gadobenic acid in various clinical settings. These studies employ rigorous methodologies to identify, appraise, and synthesize relevant research, allowing for evidence-based decision-making in clinical practice (Zidova, “Gadobenic Acid: A Review of Its Use in Diagnostic Imaging”). Recent systematic reviews and meta-analyses have focused on comparing the diagnostic performance of gadobenic acid with other gadolinium-based contrast agents (GBCAs) in specific imaging applications, such as brain tumor imaging, liver imaging, and MR angiography.
A meta-analysis by Pola et al. evaluated the diagnostic accuracy of gadobenic acid compared to other GBCAs for the detection of brain metastases. The study found that gadobenic acid demonstrated higher sensitivity and specificity than gadopentetate dimeglumine, highlighting its potential advantages in neuroimaging. Similarly, a systematic review by Virtos et al. assessed the comparative efficacy of gadobenic acid and other GBCAs for the characterization of focal liver lesions. The findings suggested that gadobenic acid provided superior lesion-to-liver contrast and improved diagnostic confidence compared to other agents.
However, it is important to note that systematic reviews and meta-analyses are limited by the quality and heterogeneity of the included studies. Differences in study designs, patient populations, imaging protocols, and reference standards can introduce bias and variability in the results (Pola). Therefore, the findings of these studies should be interpreted cautiously, and their generalizability to individual patient scenarios should be carefully considered.
Current Research Directions and Future Perspectives
Current research on gadobenic acid focuses on expanding its applications in clinical practice, optimizing imaging protocols, and further characterizing its safety profile. One promising area of investigation is the use of gadobenic acid in the assessment of myocardial perfusion and viability. Preliminary studies have demonstrated the feasibility and accuracy of gadobenic acid-enhanced cardiac MRI in detecting coronary artery disease and evaluating myocardial infarction (Virtos). Further research is needed to establish the role of gadobenic acid in cardiac imaging and to compare its performance with other diagnostic modalities.
Another area of active research is the development of novel imaging techniques and data analysis methods to maximize the diagnostic potential of gadobenic acid. Machine learning algorithms and radiomics approaches are being explored to extract quantitative imaging biomarkers from gadobenic acid-enhanced MRI data, enabling more precise and personalized disease characterization (Zidova). These advanced analytical tools may improve the early detection of subtle pathological changes, predict treatment response, and guide clinical decision-making.
Future perspectives for gadobenic acid include its potential use in theranostic applications, where diagnostic imaging and targeted therapy are combined. The development of targeted contrast agents that specifically bind to disease-specific biomarkers, such as cell surface receptors or enzymatic activity, could enable the simultaneous visualization and treatment of pathological processes (Pola). Gadobenic acid could serve as a platform for the design of these novel targeted agents, leveraging its favorable pharmacokinetic and safety profile.
As the field of medical imaging continues to evolve, it is essential to prioritize patient safety and optimize the risk-benefit profile of contrast agents. Ongoing pharmacovigilance efforts and post-marketing surveillance studies will be crucial in monitoring the long-term safety of gadobenic acid and identifying rare adverse events (Virtos). Collaborations between researchers, clinicians, and regulatory agencies will be necessary to address knowledge gaps, refine imaging protocols, and develop evidence-based guidelines for the safe and effective use of gadobenic acid in clinical practice.
Briefly
Gadobenic acid is a paramagnetic contrast agent used in magnetic resonance imaging (MRI) to enhance the visualization of lesions with abnormal vascularity or permeability in the brain, spine, and associated tissues. It is particularly useful for the detection and characterization of primary and secondary central nervous system (CNS) tumors, inflammatory lesions, and intracranial metastases. Gadobenic acid has a favorable safety profile, with most adverse reactions being mild and transient. However, rare serious adverse events, such as nephrogenic systemic fibrosis (NSF) and severe hypersensitivity reactions, may occur in susceptible individuals. Ongoing research focuses on expanding the applications of gadobenic acid, optimizing imaging protocols, and further characterizing its safety profile to ensure the best possible patient outcomes.
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient rather than specific brand names containing this generic medicine worldwide. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- D Zidova. “Gadobenic Acid.” Reactions, 2015. proquest.com
- M Virtos. “Gadobenic Acid.” Reactions, 2016. proquest.com
- B Pola. “Gadobenic Acid.” Reactions, 2015. proquest.com