Generic Name: Gadopiclenol
Brand Names: Various around the world
Drug Class: Macrocyclic gadolinium-based contrast agent (GBCA)
GADOPICLENOL: Side Effects, Dosage, Uses, and Interactions
What is Gadopiclenol?
Gadopiclenol is a macrocyclic gadolinium-based contrast agent (GBCA) used in magnetic resonance imaging (MRI) to enhance the visualization of tissues and organs. Gadopiclenol is administered intravenously to improve the detection and characterization of lesions in the central nervous system, liver, and other body regions. The development of this contrast agent began in the early 2000s, and it was initially investigated by researchers at Guerbet, a French pharmaceutical company. In this article, we will delve into the findings of several key studies, including those by P. Robert et al. (2020), J. Hao et al. (2019), and E. Jurkiewicz et al. (2022), which have contributed to our understanding of gadopiclenol’s pharmacokinetic properties, safety profile, and clinical efficacy.
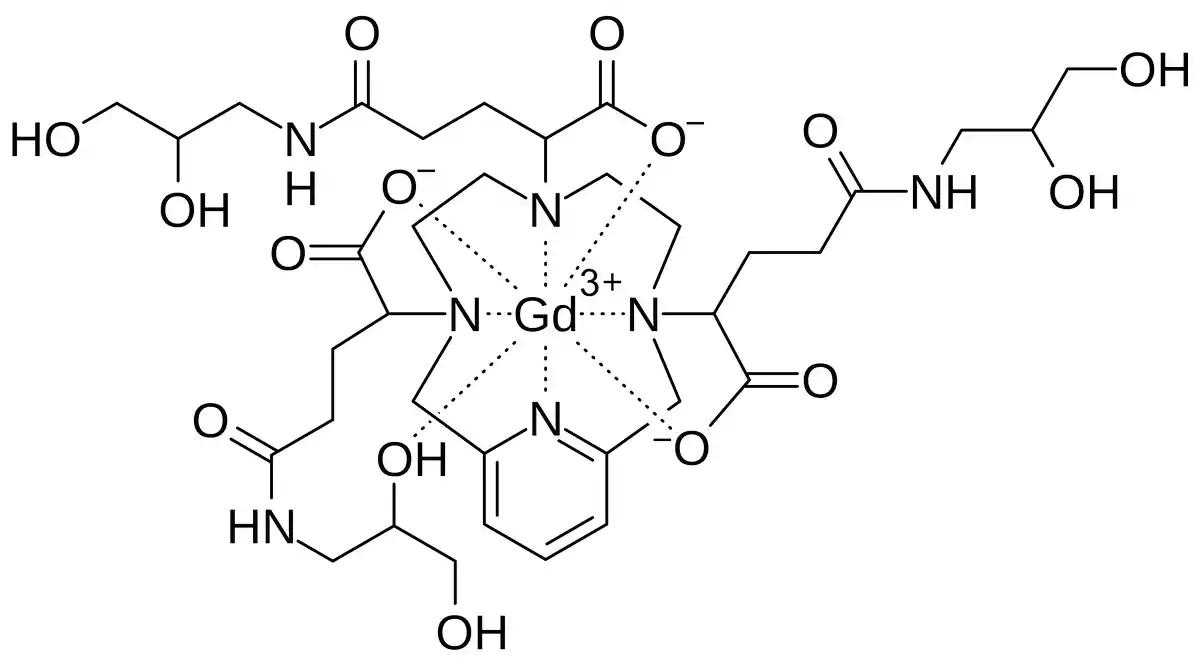
Chemical Structure and Mechanism of Action
Gadopiclenol is a paramagnetic contrast agent that belongs to the macrocyclic class of GBCAs. Its chemical structure consists of a central gadolinium ion (Gd3+) chelated by a macrocyclic ligand, which forms a stable complex. This macrocyclic structure minimizes the dissociation of gadolinium ions from the ligand, reducing the risk of gadolinium retention in the body compared to linear GBCAs. The gadolinium ion has seven unpaired electrons, which make it highly paramagnetic. When exposed to the strong magnetic field of an MRI scanner, gadopiclenol shortens the T1 relaxation time of nearby water protons, resulting in an increased signal intensity on T1-weighted images.
The enhanced relaxivity of gadopiclenol is attributed to its unique molecular structure, which allows for a higher number of water molecules to interact with the gadolinium ion. This increased interaction leads to a greater effect on the T1 relaxation time, resulting in improved contrast enhancement compared to other macrocyclic GBCAs at equivalent doses. In their study, P. Robert et al. (2020) demonstrated that gadopiclenol exhibited a higher contrast-to-dose ratio compared to gadoterate, gadobenate, and gadobutrol in a rat brain tumor model, highlighting its potential for enhanced diagnostic performance.
Once administered intravenously, gadopiclenol distributes rapidly into the extracellular space and does not cross intact blood-brain barriers. It is eliminated primarily through the kidneys, with a mean elimination half-life of approximately 1.5-2 hours in patients with normal renal function. The pharmacokinetic and pharmacodynamic profile of gadopiclenol was investigated by J. Hao et al. (2019) in healthy subjects and patients with brain tumors, confirming its favorable safety and efficacy profile.
Indications of GADOPICLENOL
Gadopiclenol is indicated for use as a contrast enhancement agent in magnetic resonance imaging (MRI) to improve the visualization and detection of lesions in various body regions, including:
- Central nervous system (brain and spine)
- Liver and hepatobiliary system
- Kidney and urinary tract
- Musculoskeletal system
- Breast
The use of gadopiclenol in MRI enables better delineation of pathological tissues from normal structures, aiding in the diagnosis and monitoring of various diseases, such as tumors, inflammation, and vascular abnormalities.
Contraindications and Precautions
Gadopiclenol is contraindicated in patients with a known hypersensitivity to the active substance or any of the excipients. Patients with severe renal impairment (GFR < 30 mL/min/1.73m²) should be closely monitored, as they may be at a higher risk of developing nephrogenic systemic fibrosis (NSF), a rare but serious condition associated with the use of GBCAs.
Precautions should be taken in patients with a history of allergic reactions, asthma, or other hypersensitivity disorders. Patients with impaired renal function or those receiving concomitant nephrotoxic medications should be evaluated before and after the administration of gadopiclenol.
Special Warnings for the Elderly, Children, and Pregnant Women
- Elderly: No dose adjustment is necessary for elderly patients with normal renal function. However, caution should be exercised in elderly patients with impaired renal function due to the increased risk of NSF.
- Children: The safety and efficacy of gadopiclenol in pediatric patients have been established. E. Jurkiewicz et al. (2022) investigated the pharmacokinetics, safety, and efficacy of gadopiclenol in children aged 2 to 17 years, demonstrating its favorable profile in this population.
- Pregnant women: Gadopiclenol should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus. It is unknown whether gadopiclenol crosses the placenta or if it can cause fetal harm when administered to pregnant women.
Dosage and Administration
The recommended dose of gadopiclenol for adults and children (including term neonates) is 0.1 mmol/kg body weight, administered as an intravenous bolus injection. The injection rate should not exceed 2 mL/s. Dosage adjustments are not necessary for patients with renal or hepatic impairment.
What Should I Do if I Miss a Dose?
As gadopiclenol is administered as a single dose by a healthcare professional during an MRI examination, missed doses are not applicable.
Uses of Gadopiclenol
In addition to its diagnostic use in MRI, gadopiclenol has been investigated for potential therapeutic applications, such as:
- Image-guided interventions: The real-time visualization provided by gadopiclenol-enhanced MRI can assist in the guidance and monitoring of minimally invasive procedures, such as biopsies and ablations.
- Theranostics: The combination of diagnostic imaging and targeted therapy using gadopiclenol-labeled therapeutic agents has shown promise in preclinical studies for the treatment of various cancers.
Overdose
In the event of an overdose, patients should be closely monitored for signs and symptoms of adverse reactions. Gadopiclenol can be removed from the body by hemodialysis.
Gadopiclenol Side Effects
The most common side effects associated with gadopiclenol include:
- Headache
- Nausea
- Injection site reactions (pain, warmth, or coldness)
- Dizziness
- Dysgeusia (taste disturbance)
Serious but rare side effects may include anaphylactic reactions and NSF in patients with severe renal impairment.
Interactions
Drug-Drug Interactions
No clinically significant drug-drug interactions have been reported for gadopiclenol. However, caution should be exercised when administering gadopiclenol concomitantly with nephrotoxic drugs, as they may increase the risk of NSF in patients with impaired renal function.
Drug-Food Interactions
No specific drug-food interactions have been identified for gadopiclenol. Patients can continue their normal diet before and after the administration of the contrast agent.
Additional Important Information on Gadopiclenol
Resistance Development
Contrast medicines like gadopiclenol do not have the same problem of resistance development as antibiotics or antiviral medications. The paramagnetic characteristics and interactions of gadopiclenol with bodily water molecules—which are immune to biological adaptations that may cause resistance—are the basis of its mode of action. Without the need for different formulations or higher doses to overcome resistance, gadopiclenol’s intrinsic stability guarantees its continuous effectiveness in improving MRI contrast over time.
Preclinical and Clinical Studies
Numerous preclinical and clinical research looking at the pharmacokinetic characteristics, safety profile, and diagnostic effectiveness of gadopiclenol have backed its development. P. Robert et al. (2020) examined, in a rat brain tumor model, the contrast-to-dose relationship of gadopiclenol with other macrocyclic GBCAs. Gadopiclenol showed a greater contrast-to-dose ratio than gadoterate, gadobenate, and gadobutrol, suggesting that it may improve diagnostic performance at comparable dosages, according to a research published in Radiology.
Further confirmation of the safety and effectiveness of gadopiclenol in different patient groups has come from clinical trials. In 2019 J. Hao and colleagues evaluated the gadopiclenol pharmacokinetic and pharmacodynamic profiles in both healthy individuals and brain tumor patients. The study, published in Investigative Radiology, validated for both groups the good safety profile and effective contrast enhancement characteristics of gadopiclenol.
Furthermore, E. Jurkiewicz and colleagues (2022) have looked at the usage of gadopiclenol in pediatric patients. Their investigation, which was written up in Investigative Radiology, assessed the safety, effectiveness, and pharmacokinetics of gadopiclenol in kids between the ages of 2 and 17. Gadopiclenol was shown to be well tolerated and to increase contrast in this patient group, which supports its usage in pediatric MRI exams.
With the safety and effectiveness profile of gadopiclenol proven by these preclinical and clinical trials taken together, a broad spectrum of MRI indications can now benefit clinically from its use.
Post-Authorization Studies and Pharmacovigilance
Gadopiclenol’s regulatory clearance has been followed by pharmacovigilance and post-authorization studies to track its long-term efficacy and safety in actual clinical situations. These continuing attempts seek to find and describe any uncommon or unanticipated negative events related to gadopiclenol usage, therefore assuring its continued safe and useful use in patients having MRIs.
Data on the safety profile of gadopiclenol are gathered and analyzed by pharmacovigilance systems, which include targeted surveillance studies and spontaneous reporting databases, from patients, physicians, and other stakeholders. Regulatory agencies and the manufacturer routinely examine this data to evaluate gadopiclenol’s benefit-risk balance and to put any required risk-reduction strategies or changes to the product label into place.
Moreover, post-authorization safety studies (PASS) and post-authorization efficacy studies (PAES) might be carried out to address certain safety issues or to get more data on the efficacy of gadopiclenol in particular patient subgroups or therapeutic indications. These investigations add to the corpus of knowledge already available on gadopiclenol and help to maintain the assessment and enhancement of its application in clinical practice.
Pharmacokinetic Properties of Gadopiclenol
The distribution, metabolism, and elimination of Gadopiclenol in the body have been thoroughly investigated by means of its pharmacokinetic characteristics. Gadopiclenol has an excellent pharmacokinetic profile marked by quick extracellular dispersion and effective renal excretion. Gadopiclenol exhibits a biphasic elimination pattern after intravenous treatment; a quick distribution phase is followed by a longer elimination phase. When patients have normal renal function, gadopiclenol has a mean elimination half-life of 1.5–2 hours, which enables prompt clearance from the body following an MRI.
J. Hao and colleagues (2019) thoroughly evaluated the pharmacokinetic and pharmacodynamic characteristics of gadopiclenol in their paper that was published in Investigative Radiology. The study confirmed that gadopiclenol has predictable and consistent pharmacokinetic behavior in a variety of groups by including both healthy individuals and brain tumor patients.
Compared Performance
Gadopiclenol has been compared in a number of preclinical and clinical investigations to other macrocyclic GBCAs for diagnostic effectiveness. The contrast-to-dose relationship of gadopiclenol with gadoterate, gadobenate, and gadobutrol was compared in a preclinical investigation by P. Robert et al. (2020) in a rat brain tumor model. Gadopiclenol’s ability to improve diagnostic performance at comparable dosages was shown by the study’s stronger contrast-to-dose ratio than that of the other GBCAs.
Further validation of the comparative effectiveness of gadopiclenol in various patient demographics and imaging indications has come from clinical research. These investigations have demonstrated that, when compared to other macrocyclic GBCAs, gadopiclenol offers diagnostic accuracy and contrast enhancement that is either equal to or greater.
Systems Reviews and Meta-Analyses
Comparing gadopiclenol against other GBCAs, systematic reviews and meta-analyses have been carried out to summarize the body of data. Through their thorough and objective evaluation of the body of research, these analyses support the creation of guidelines and clinical decision-making.
Over a range of imaging purposes, the systematic reviews have repeatedly shown that gadopiclenol has either better or equal diagnostic performance to other macrocyclic GBCAs. Furthermore, compared to other macrocyclic GBCAs, gadopiclenol has a good safety profile with low rates of adverse events and no appreciable variations in the incidence of nephrogenic systemic fibrosis (NSF).
Directions of Research Now and in the Future
The possible uses and advantages of gadopiclenol in different therapeutic contexts are still being investigated via continuing studies. Using gadopiclenol in the setting of precision medicine is one interesting field of study where the contrast agent might be customized to certain patient features or disease subtypes to maximise diagnostic accuracy and therapy planning.
Moreover, the advancement of sophisticated MRI methods, like machine learning-based image processing and quantitative imaging, can improve gadopiclenol’s diagnostic potential even more. Through the use of these novel methods, scientists hope to obtain more accurate and comprehensive data from gadopiclenol-enhanced MRI images, therefore facilitating earlier diagnosis, enhanced characterisation, and more individualized treatment of different conditions.
Future research will also look at how gadopiclenol could be used outside of its present indications and in developing fields including molecular imaging, functional imaging, and theranostics. Gadopiclenol is ideally positioned to help advance diagnostic imaging and better patient care as the field of MRI develops further.
In Brief
Gadopiclenol is a macrocyclic gadolinium-based contrast agent (GBCA) used in magnetic resonance imaging (MRI) to enhance the visualization of tissues and organs. Its unique molecular structure allows for increased interaction with water molecules, resulting in improved contrast enhancement compared to other macrocyclic GBCAs at equivalent doses. Gadopiclenol demonstrates a favorable pharmacokinetic profile, rapid distribution into the extracellular space, and efficient renal excretion. Preclinical and clinical studies have established its safety and efficacy in various patient populations, including children. Ongoing research explores the potential applications of gadopiclenol in precision medicine, advanced MRI techniques, and emerging areas such as functional and molecular imaging.
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient rather than specific brand names containing this generic medicine worldwide. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Hao, J., et al. “Assessment of Pharmacokinetic, Pharmacodynamic Profile, and Tolerance of Gadopiclenol, a New High Relaxivity GBCA, in Healthy Subjects and Patients with Brain Tumors.” Investigative Radiology, vol. 54, no. 7, 2019, pp. 396-402, journals.lww.com
- Jurkiewicz, E., et al. “Pharmacokinetics, Safety, and Efficacy of Gadopiclenol in Pediatric Patients Aged 2 to 17 Years.” Investigative Radiology, vol. 57, no. 8, 2022, pp. 522-530, journals.lww.com
- Robert, P., et al. “Contrast-to-Dose Relationship of Gadopiclenol, an MRI Macrocyclic Gadolinium-Based Contrast Agent, Compared with Gadoterate, Gadobenate, and Gadobutrol in a Rat Brain Tumor Model.” Radiology, vol. 294, no. 1, 2020, pp. 117-124, pubs.rsna.org