Brand Names: Various around the world
Drug Class: Opioid partial agonist
Buprenorphine Side Effects, Uses, Dosage, Interactions, Warnings, and Overdose
What is Buprenorphine?
Buprenorphine is a semisynthetic opioid medication primarily used to treat opioid use disorder. It is also employed for pain management, particularly in cases of moderate to severe chronic pain. Buprenorphine helps manage withdrawal symptoms and cravings in individuals dependent on opioids, making it a crucial tool in addiction treatment.
Mechanism of action: Buprenorphine acts as a partial agonist at mu-opioid receptors and an antagonist at kappa-opioid receptors. This unique pharmacological profile contributes to its effectiveness in managing opioid dependence while potentially offering a lower risk of respiratory depression compared to full opioid agonists.
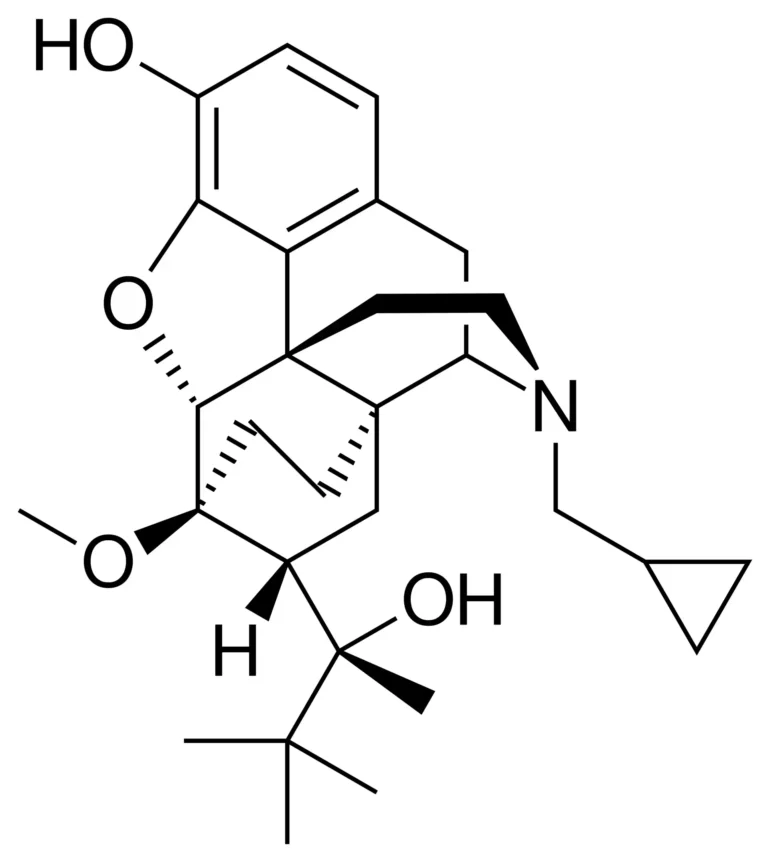
Chemical structure: C29H41NO4
Therapeutic category: Opioid partial agonist for addiction treatment and pain management.
Buprenorphine is available in various formulations, including sublingual tablets, buccal films, transdermal patches, and injectable forms. The choice of formulation depends on the specific indication and patient needs. Its long duration of action allows for less frequent dosing compared to some other opioid medications, potentially improving treatment adherence.
History of Medicine
Buprenorphine was first synthesized in 1966 by researchers at Reckitt & Colman (now Reckitt Benckiser) in the United Kingdom. The drug was initially developed as a pain medication, derived from thebaine, an alkaloid of the poppy Papaver somniferum. Dr. John Lewis and his team led the research efforts that resulted in buprenorphine’s creation.
In the 1970s, animal studies revealed buprenorphine’s potential for treating opioid addiction. This discovery led to human trials in the 1980s, which confirmed its efficacy in managing opioid dependence. The U.S. Food and Drug Administration (FDA) approved buprenorphine for pain management in 1981.
A significant milestone occurred in 2002 when the FDA approved buprenorphine (Subutex) and buprenorphine/naloxone combination (Suboxone) for the treatment of opioid dependence. This approval, under the Drug Addiction Treatment Act of 2000, allowed for the medication to be prescribed in office-based settings, greatly expanding access to opioid addiction treatment.
Recent research on buprenorphine has been published in prestigious medical journals such as the New England Journal of Medicine and Annals of Emergency Medicine. These studies have explored its efficacy in various clinical settings, particularly in comparison to methadone for treating opioid use disorder during pregnancy and optimizing induction protocols.
Anatomical/therapeutic/chemical (ATC) classification
ATC Code: N07BC01 Title: Buprenorphine
Classification: Nervous system; Other nervous system drugs; Drugs used in addictive disorders; Drugs used in opioid dependence
Indications of Buprenorphine
Mostly suggested for the treatment of opioid use disorder is bupenorphine. In those hooked on opioids, it helps control cravings and withdrawal symptoms. Furthermore recommended for pain treatment are several formulations, especially in situations of moderate to severe chronic pain.
Contraindications and Precautions
Buprenorphine is contraindicated in patients with:
- Known hypersensitivity to buprenorphine or any components of the formulation
- Severe respiratory depression
- Severe hepatic impairment
- Acute alcoholism or delirium tremens
Caution is advised in patients with:
- Compromised respiratory function
- Head injury or increased intracranial pressure
- Liver or kidney disease
- History of seizures
- Biliary tract dysfunction
Special Warnings for the Elderly, Children and Pregnant Women
Elderly people might need dosage changes and be more susceptible to the effects of buprenorphine. Children under sixteen should not take buprenorphine unless directed directly by a doctor.
Only if the possible benefit justifies the possible harm to the baby should pregnant women take buprenorphine. “buprenorphine use in pregnancy was associated with a lower risk of adverse neonatal outcomes than methadone use,” according a recent research by Suarez et al. that was written up in the New England Journal of Medicine. Still, newborn withdrawal syndrome may strike.
Dosage and Administration
Dosage varies based on the specific formulation and indication. For opioid use disorder treatment, typical sublingual doses range from 4-24 mg daily. Transdermal patches for pain management are usually changed every 7 days.
What Should I Do If I Miss a Dose?
If a dose is missed, take it as soon as remembered. However, if it’s almost time for the next scheduled dose, skip the missed dose and resume the regular dosing schedule. Do not double up on doses.
Uses of Buprenorphine
Beyond its primary use in opioid addiction treatment, buprenorphine has shown efficacy in:
- Chronic pain management
- Acute pain relief in certain settings
- Treatment of depression (investigational)
Overdose
Buprenorphine overdose can cause severe respiratory depression, sedation, and potentially death. Symptoms may include:
- Pinpoint pupils
- Extreme drowsiness
- Slowed breathing
- Loss of consciousness
Immediate medical attention is crucial. Naloxone can reverse buprenorphine’s effects, but higher doses may be required compared to other opioids.
Interactions
Drug-Drug Interactions
Buprenorphine can interact with various medications:
- Benzodiazepines and other CNS depressants: Increased risk of respiratory depression
- CYP3A4 inhibitors (e.g., ketoconazole, ritonavir): May increase buprenorphine levels
- CYP3A4 inducers (e.g., rifampin, carbamazepine): May decrease buprenorphine efficacy
- Other opioids: Potential for precipitated withdrawal
Drug-Food Interactions
Grapefruit juice may increase buprenorphine levels by inhibiting CYP3A4 metabolism. Alcohol should be avoided due to increased risk of respiratory depression and sedation.
Greenwald et al. in their study published in Annals of Emergency Medicine propose that “the acute clinical success of buprenorphine induction (achieving a positive agonist-to-withdrawal balance) is a nonlinear outcome of the opioid balance at the time of initial buprenorphine dose and mu-opioid–receptor affinity, lipophilicity, and mu-opioid–receptor intrinsic efficacy” (Greenwald et al.). This underscores the complexity of buprenorphine’s interactions and the importance of careful dosing and monitoring.
Buprenorphine Side Effects
While buprenorphine is generally well-tolerated, it can cause various side effects. The frequency and severity of these effects often depend on factors such as dosage, duration of use, and individual patient characteristics.
Common side effects include:
- Nausea and vomiting
- Constipation
- Headache
- Dizziness
- Drowsiness
- Sweating
- Dry mouth
These effects typically subside as the body adjusts to the medication. However, persistent or severe symptoms should be reported to a healthcare provider.
Rare but Possible Buprenorphine Side Effects
Less common side effects may include:
- Insomnia
- Anxiety
- Mood changes
- Muscle aches
- Abdominal pain
- Decreased libido
- Hair loss
In their study, Suarez et al. noted that “the risk of adverse maternal outcomes was similar among persons who received buprenorphine and those who received methadone” (Suarez et al.). This suggests that while side effects occur, they may not be significantly different from other opioid treatments.
Greenwald et al. highlight that “buprenorphine causes minimal mu-opioid–receptor desensitization or internalization and can suppress its precipitated withdrawal“ (Greenwald et al.). This unique pharmacological profile may contribute to its generally favorable side effect profile compared to full opioid agonists.
How to Manage Buprenorphine Side Effects
Managing buprenorphine side effects often involves a combination of lifestyle adjustments and medical interventions:
- Nausea and vomiting: • Take medication with food • Stay hydrated • Consider anti-emetic medications if prescribed
- Constipation: • Increase fiber intake • Stay hydrated • Regular exercise • Stool softeners or laxatives if recommended by a healthcare provider
- Headache and dizziness: • Rest in a quiet, dark room • Ensure proper hydration • Over-the-counter pain relievers if approved by a doctor
- Drowsiness: • Avoid driving or operating machinery until effects are known • Adjust dosing schedule if possible (e.g., taking at bedtime)
- Sweating: • Stay hydrated • Wear breathable clothing • Consider antiperspirants
For serious side effects, immediate medical attention is crucial. Patients should be educated about potential warning signs and when to seek emergency care.
Chambers et al. found that “patients prescribed a 24 mg dose of buprenorphine remained in treatment longer than those prescribed 16 mg” (Chambers et al.). This suggests that higher doses may be more effective in some cases, but also underscores the importance of individualized dosing to balance efficacy and side effects.
Ongoing communication with healthcare providers is essential for managing side effects effectively. Patients should report any persistent or concerning symptoms promptly. In some cases, dosage adjustments or changes in formulation may be necessary to optimize treatment while minimizing adverse effects.
Additional Important Information of Buprenorphine
Resistance Development
With a different pharmacological profile than complete opioid agonists, burenorphine may have benefits in terms of resistance development. Buprenorphine has a ceiling impact on respiratory depression as a partial mu-opioid receptor agonist, therefore maybe lowering the danger of tolerance to its respiratory depressing action. Long-term usage still allows tolerance to its analgesic effects, however.
A complicated phenomena is the development of resistance to buprenorphine’s therapeutic benefits in treatment of opioid use disorder. Although certain patients may need dosage changes over time, the medication’s great affinity for mu-opioid receptors and moderate dissociation rate help to explain its long-lasting effects and maybe reduced chance of resistance development compared to other opioids.
Preclinical and Clinical Studies
Preclinical studies have elucidated buprenorphine’s unique pharmacological properties. Research has demonstrated its partial agonist activity at mu-opioid receptors and antagonist activity at kappa-opioid receptors, contributing to its efficacy in both pain management and addiction treatment.
Clinical studies have further substantiated buprenorphine’s effectiveness. In their research published in the Annals of Emergency Medicine, Greenwald et al. propose a neuropharmacological model to explain buprenorphine induction challenges. They suggest that “the acute clinical success of buprenorphine induction (achieving a positive agonist-to-withdrawal balance) is a nonlinear outcome of the opioid balance at the time of initial buprenorphine dose and mu-opioid–receptor affinity, lipophilicity, and mu-opioid–receptor intrinsic efficacy” (Greenwald et al.). This model provides valuable insights into optimizing buprenorphine induction protocols.
Post-authorization Studies and Pharmacovigilance
Post-authorization studies have continued to assess buprenorphine’s safety and efficacy in real-world settings. These studies have been crucial in identifying rare adverse effects and evaluating long-term outcomes. Pharmacovigilance efforts have focused on monitoring potential misuse, diversion, and unintended consequences of buprenorphine treatment.
A significant area of post-authorization research has been the use of buprenorphine in special populations. Suarez et al., in their study published in the New England Journal of Medicine, investigated buprenorphine use in pregnancy. Their findings indicated that “buprenorphine use in pregnancy was associated with a lower risk of adverse neonatal outcomes than methadone use” (Suarez et al.). This research has important implications for treating opioid use disorder in pregnant individuals.
Pharmacokinetic Characteristics of Buprenorphine
Buprenorphine exhibits complex pharmacokinetics. It undergoes extensive first-pass metabolism in the liver, primarily via CYP3A4 enzymes. The medication has high lipophilicity, allowing for various administration routes including sublingual, transdermal, and parenteral.
The drug’s long half-life contributes to its extended duration of action. Buprenorphine’s high affinity for mu-opioid receptors results in prolonged receptor occupancy, even at low plasma concentrations. This characteristic underlies its effectiveness in managing opioid dependence and withdrawal symptoms.
Current Research Directions and Future Perspectives
Current research on buprenorphine is focused on several key areas:
- Optimizing dosing regimens: Studies like the one conducted by Chambers et al. are exploring the impact of different dosing strategies on treatment outcomes.
- Novel formulations: Research is ongoing into long-acting injectable and implantable forms of buprenorphine to improve treatment adherence.
- Combination therapies: Investigations into combining buprenorphine with other medications to enhance its efficacy or mitigate side effects are underway.
- Expanding indications: Researchers are exploring buprenorphine’s potential in treating other conditions, such as treatment-resistant depression.
- Personalized medicine approaches: Studies are aiming to identify genetic or clinical factors that may predict individual responses to buprenorphine treatment.
Effectiveness
It is indisputable that buprenorphine helps treat opioid use disorder. Among those with opioid dependency, it has been proven to lower death rates, enhance treatment retention, and cut illegal opioid usage. Particularly in relation to respiratory depression risk, the partial agonistic qualities of the drug help to explain its good safety profile.
In pain treatment, buprenorphine has shown success treating both acute and chronic pain disorders. Its special pharmacology might provide benefits in certain patient groups, including those with renal disease or high risk of opioid-induced respiratory depression.
Comparative Efficacy, Systematic Reviews and Meta-analyses
Meta-analyses and systematic reviews repeatedly show buprenorphine’s effectiveness in treating opioid use disorders. Comparisons with another often used drug for opioid dependency, methadone, have shown conflicting findings. While some research indicate similar effectiveness, others have shown variations in particular results.
For pregnant women with opioid use disorder, the study by Suarez et al. offers insightful analysis of the relative effectiveness of buprenorphine and methadone. Their results imply possible benefits of buprenorphine in terms of newborn results, therefore supporting the continuous debate on the best treatment strategies in this group.
Future studies will surely keep improving our knowledge of buprenorphine’s position in the armamentarium of therapy for pain management and opioid use disorder. Effective, safe, and easily available medicines like buprenorphine remain most important as the opioid epidemic continues.
Scientific Research
Analysis of the Research Study “Buprenorphine versus Methadone for Opioid Use Disorder in Pregnancy”
This groundbreaking study, conducted by Elizabeth A. Suarez, Krista F. Huybrechts, Loreen Straub, Sonia Hernández-Díaz, Hendrée E. Jones, Hilary S. Connery, Jonathan M. Davis, Kathryn J. Gray, Barry Lester, Mishka Terplan, Helen Mogun, and Brian T. Bateman, provides crucial insights into the comparative efficacy and safety of buprenorphine and methadone for treating opioid use disorder during pregnancy.
Study Design and Methodology:
The researchers employed a cohort study design, analyzing data from pregnant individuals enrolled in public insurance programs in the United States from 2000 to 2018. This extensive timeframe and large-scale data source allowed for a comprehensive examination of outcomes associated with buprenorphine and methadone use during pregnancy.
Key methodological strengths include:
- Large Sample Size: The study included data from 2,548,372 pregnancies that ended in live births, with 10,704 exposed to buprenorphine and 4,387 to methadone in early pregnancy.
- Propensity Score Matching: To control for potential confounding factors, the researchers used propensity-score overlap weights, enhancing the validity of their comparisons.
- Multiple Exposure Windows: The study assessed exposure during early pregnancy, late pregnancy, and the 30 days before delivery, providing a nuanced understanding of medication effects throughout gestation.
Key Findings:
The study revealed several significant outcomes:
- Neonatal Abstinence Syndrome (NAS): Infants exposed to buprenorphine in the 30 days before delivery had a lower incidence of NAS compared to those exposed to methadone (52.0% vs. 69.2%, adjusted relative risk: 0.73).
- Preterm Birth: Buprenorphine exposure in early pregnancy was associated with a lower risk of preterm birth compared to methadone exposure (14.4% vs. 24.9%, adjusted relative risk: 0.58).
- Birth Weight: Infants exposed to buprenorphine had a lower risk of low birth weight compared to those exposed to methadone (8.3% vs. 14.9%, adjusted relative risk: 0.56).
- Maternal Outcomes: The risks of cesarean section and severe maternal complications were similar between the buprenorphine and methadone groups.
Implications and Significance:
This research has substantial implications for clinical practice and policy:
- Treatment Selection: The findings support the use of buprenorphine as a first-line treatment for opioid use disorder in pregnancy, given its more favorable neonatal outcomes.
- Clinical Guidelines: The study may influence updates to clinical guidelines for managing opioid use disorder during pregnancy.
- Patient Counseling: Healthcare providers can use this information to better inform pregnant patients about the potential risks and benefits of buprenorphine versus methadone treatment.
- Health Policy: The results may impact health insurance coverage decisions and public health strategies for addressing opioid use disorder in pregnant populations.
Limitations and Future Directions:
While robust, the study has some limitations:
- Observational Design: As an observational study, it cannot definitively establish causality.
- Potential Confounding: Despite rigorous statistical methods, unmeasured confounding factors may still influence the results.
- Generalizability: The study population was limited to those enrolled in public insurance programs, potentially limiting generalizability to other populations.
Future research directions suggested by this study include:
- Prospective, randomized controlled trials to further validate these findings.
- Investigation of long-term outcomes for children exposed to these medications in utero.
- Exploration of potential mechanisms underlying the observed differences in outcomes between buprenorphine and methadone.
Ultimately, especially in terms of newborn outcomes, this thorough research by Suarez et al. offers convincing evidence for the possible benefits of buprenorphine over methadone in treating opioid use disorder during pregnancy. The study is a major addition to the field as it provides insightful direction for doctors and legislators on handling the difficult junction of opiate use disorder and pregnancy.
Briefly
Mostly used to treat opioid use disorder, buprenorphine is a semisynthetic opioid drug. In those dependent on opioids, it helps control cravings and withdrawal symptoms as a partial agonist at mu-opioid receptors. With a ceiling impact on respiratory depression, buprenorphine has a different pharmacological profile than other opioids and could provide a safer substitute. Recent studies on its effectiveness in many therapeutic environments, including pregnancy, where it has demonstrated encouraging outcomes relative to methadone, have Like other opioids, buprenorphine does, however, have adverse effects and potential for abuse, hence, strict medical monitoring and tailored treatment plans are even more important.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Chambers, L. C., et al. “Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl.” JAMA Network Open, 2023. jamanetwork.com
- Greenwald, M. K., et al. “A Neuropharmacological Model to Explain Buprenorphine Induction Challenges.” Annals of Emergency Medicine, 2022. sciencedirect.com
- Suarez, E. A., et al. “Buprenorphine versus methadone for opioid use disorder in pregnancy.” New England Journal of Medicine, 2022. nejm.org
FAQ
What is buprenorphine and how does it work?
Buprenorphine is a partial opioid agonist used to treat opioid use disorder and pain. It binds to opioid receptors, reducing cravings and withdrawal symptoms. As a partial agonist, it has a ceiling effect on respiratory depression, potentially offering a safer profile than full opioid agonists. Always consult a doctor for personalized medical advice.
What are common buprenorphine side effects?
Common side effects include nausea, constipation, headache, dizziness, and drowsiness. Some patients may experience sweating, dry mouth, or insomnia. These effects often subside as the body adjusts to the medication. However, persistent or severe symptoms should be reported to a healthcare provider immediately.
How does buprenorphine/naloxone combination therapy work?
Buprenorphine/naloxone combines buprenorphine with naloxone, an opioid antagonist. This formulation helps deter misuse, as naloxone can precipitate withdrawal if the medication is injected. When taken as prescribed sublingually, naloxone has minimal effect. A doctor can provide more detailed information about this treatment approach.
What is a buprenorphine patch used for?
Buprenorphine patches are primarily used for pain management, particularly in cases of moderate to severe chronic pain. The patch delivers a continuous, controlled dose of buprenorphine through the skin. It's often prescribed when other pain medications have proven ineffective or unsuitable. Always follow a doctor's instructions when using transdermal medications.
Can buprenorphine be used for cats?
Yes, veterinarians sometimes prescribe buprenorphine for pain management in cats. It's used for both acute and chronic pain conditions in felines. The dosage and administration method for cats differ significantly from human use. Never give human medications to pets without veterinary guidance, as it can be dangerous.
What is the typical dosage for buprenorphine?
Buprenorphine dosage varies based on the specific formulation, indication, and individual patient factors. For opioid use disorder, sublingual doses typically range from 4-24 mg daily. Pain management doses differ. A healthcare provider will determine the appropriate dosage based on a patient's specific needs and medical history.
How long does buprenorphine stay in your system?
Buprenorphine's effects can last 24-72 hours, but it may be detectable in urine tests for up to 2 weeks after the last dose. Factors like dosage, frequency of use, metabolism, and individual physiology affect its duration in the body. For accurate information about detection times, consult a healthcare professional.
What are the brand names for buprenorphine?
Common brand names for buprenorphine include Subutex (buprenorphine alone) and Suboxone (buprenorphine/naloxone combination). Other brands include Zubsolv, Bunavail, and Belbuca. The transdermal patch is marketed as Butrans. Availability may vary by country. A healthcare provider can discuss which formulation is most appropriate for individual needs.
Is buprenorphine addictive?
While buprenorphine has a lower potential for misuse than full opioid agonists, it can still be addictive. Its partial agonist properties and ceiling effect on euphoria reduce, but don't eliminate, addiction risk. When used as prescribed under medical supervision for opioid use disorder treatment, the benefits often outweigh the risks. Always follow a doctor's guidance.