Generic Name: Acyclovir
Brand Names: Various around the world
Drug Class: Antiviral
Acyclovir Side Effects, Uses, Dosage, Interactions, Warnings, and Overdose.
What is Acyclovir?
Acyclovir is an antiviral medication used to treat infections caused by herpes simplex viruses (HSV-1 and HSV-2), varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). It is commonly prescribed for conditions such as genital herpes, cold sores, shingles, and chickenpox. Acyclovir works by inhibiting viral DNA replication, thereby preventing the spread of the infection.
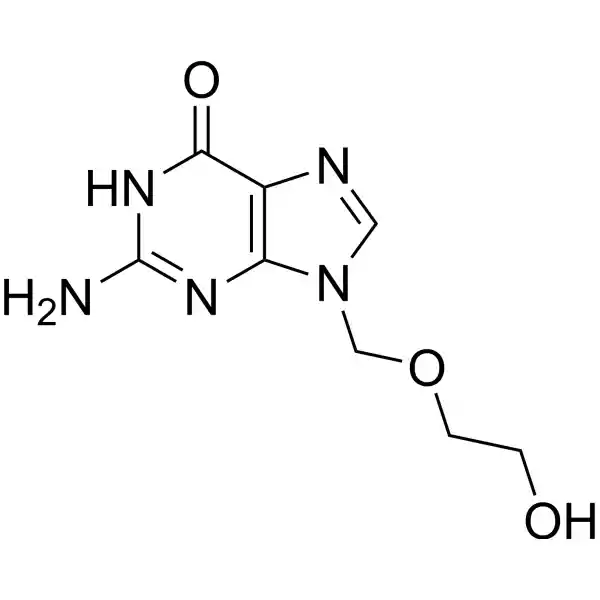
Mechanism of action: Acyclovir is selectively phosphorylated by viral thymidine kinase to its active form, acyclovir triphosphate. This active metabolite competitively inhibits viral DNA polymerase, leading to premature chain termination during viral DNA synthesis.
Chemical structure: Acyclovir is a guanosine analogue with a modified sugar moiety.
Therapeutic category: Antiviral agent
History of Medicine
Howard Schaeffer and colleagues at Burroughs Wellcome Co. (now part of GlaxoSmithKline) first synthesized acyclovir in 1974. Schaeffer and Gertrude Elion worked together to produce it; subsequently, her work on medication development helped her to win the Nobel Prize in Physiology or Medicine. A major development in antiviral treatment came when the FDA authorized accyclovir in 1982 to treat herpes infections.
Acyclovir’s invention transformed the way herpes virus infections were treated and offered a better, safer substitute for earlier treatments. Its popularity generated a new paradigm for focused drug design and helped subsequent antiviral drugs to be developed.
In this article, we will analyze recent research on acyclovir, including a scoping review by Aboelezz and Mahmoud on acyclovir dosing in herpes encephalitis, as well as a study by Schalkwijk et al. on acyclovir resistance in herpes simplex viruses.
Anatomical/therapeutic/chemical (ATC) classification
ATC Code: J05AB01 Title: Acyclovir Classification: Antivirals for systemic use, Direct acting antivirals, Nucleosides and nucleotides excluding reverse transcriptase inhibitors
Indications of Acyclovir
Acyclovir is primarily prescribed for:
- Treatment of herpes simplex virus (HSV) infections, including genital herpes and cold sores
- Management of varicella-zoster virus (VZV) infections, such as chickenpox and shingles
- Prevention of HSV infections in immunocompromised patients
- Treatment of herpes simplex encephalitis
In severe cases like herpes encephalitis, intravenous acyclovir is the treatment of choice. As noted by Aboelezz and Mahmoud, “Acyclovir greatly decreases the morbidity and mortality of herpes encephalitis if it is started immediately.”
Contraindications and Precautions
Acyclovir is contraindicated in patients with known hypersensitivity to the drug or its components. Caution is advised in:
- Patients with renal impairment
- Those with neurological abnormalities
- Individuals with hepatic dysfunction
Monitoring renal function is crucial, especially in patients receiving high doses or those with pre-existing kidney issues.
Special Warnings for the Elderly, Children, and Pregnant Women
- Elderly: Dose adjustment may be necessary due to decreased renal function. Close monitoring is essential.
- Children: Acyclovir is generally safe for use in children, but dosage should be carefully calculated based on body weight.
- Pregnant women: While acyclovir is considered relatively safe during pregnancy (Category B), it should be used only when clearly needed. The potential benefits must outweigh possible risks.
Dosage and Administration
Dosage varies depending on the indication, severity of infection, and patient characteristics. For herpes encephalitis, Aboelezz and Mahmoud state: “The recommended dose of acyclovir in severe infections such as herpetic encephalitis is 10mg/kg intravenous (IV) every 8 hours in normal kidney function to be given for 14–21 days.”
For other indications:
- Genital herpes: 400 mg orally three times daily for 7-10 days
- Cold sores: 400 mg orally five times daily for 5 days
- Shingles: 800 mg orally five times daily for 7-10 days
Dosage adjustments are necessary for patients with renal impairment.
What Should I Do If I Miss a Dose?
If a dose is missed, take it as soon as remembered. However, if it’s close to the time for the next dose, skip the missed dose and resume the regular dosing schedule. Do not double up on doses to make up for a missed one.
Uses of Acyclovir
Beyond its primary indications, acyclovir has shown efficacy in:
- Preventing HSV reactivation in patients undergoing dental procedures
- Managing herpetic whitlow (finger infections)
- Treating herpes gladiatorum in athletes
Overdose
Acyclovir overdose can lead to serious consequences, including:
- Acute renal failure
- Neurological symptoms (confusion, hallucinations, seizures)
- Gastrointestinal disturbances
Immediate medical attention is crucial in cases of suspected overdose. Treatment typically involves supportive care and, if necessary, hemodialysis to remove excess drug from the system.
Interactions
Drug-Drug Interactions
Acyclovir may interact with several medications:
- Probenecid: Can increase acyclovir plasma concentrations
- Mycophenolate mofetil: May reduce the effectiveness of acyclovir
- Nephrotoxic drugs: Concurrent use may increase the risk of kidney damage
Schalkwijk et al. highlight the importance of considering drug interactions, particularly in immunocompromised patients who may be on multiple medications.
Drug-Food Interactions
While acyclovir can generally be taken with or without food, high-fat meals may slightly decrease its absorption. Maintaining adequate hydration is important to minimize the risk of kidney-related side effects.
Acyclovir Side Effects
While acyclovir is generally well-tolerated, it can cause various side effects. Understanding these potential reactions is crucial for patients and healthcare providers alike.
Common side effects of acyclovir include:
- Nausea and vomiting
- Headache
- Diarrhea
- Dizziness
- Fatigue
These effects are typically mild and often resolve on their own. However, persistent or severe symptoms should be reported to a healthcare professional.
Rare but Possible Acyclovir Side Effects
Less frequently, patients may experience:
- Skin rashes or itching
- Temporary hair loss
- Changes in vision
- Joint pain
- Swelling of the hands, feet, or ankles
These side effects, while uncommon, warrant medical attention if they occur or persist.
Healthcare providers should be vigilant for these rare but serious side effects, especially in high-risk patients or those receiving high doses of acyclovir.
How to Manage Acyclovir Side Effects
Managing acyclovir side effects involves a combination of preventive measures and prompt intervention:
- Proper hydration: Maintaining adequate fluid intake can help prevent kidney-related side effects.
- Dose adjustment: For patients with renal impairment, dosage modifications may be necessary to prevent toxicity.
- Monitoring: Regular assessment of renal function and neurological status is crucial, especially in high-risk patients.
- Patient education: Informing patients about potential side effects and when to seek medical attention is essential.
- Prompt discontinuation: In cases of severe reactions, immediately stopping acyclovir and seeking medical care is vital.
For neurotoxicity, Aboelezz and Mahmoud suggest that “measuring the concentration of acyclovir as well as its metabolite is important for optimizing the therapeutic outcomes.” In some cases, hemodialysis may be necessary to rapidly reduce acyclovir levels and alleviate symptoms.
It is noteworthy that the advantages of acyclovir in treating herpes infections usually exceed the adverse effect dangers. Still, safe and efficient therapy depends on honest communication between patients and doctors. Any odd symptoms or worries should be reported at away to guarantee patient safety and best therapy.
Additional Important Information of Acyclovir
Resistance Development
Antiviral treatment is much challenged by the development of acyclovir-resistant herpes simplex virus (HSV) strains. While acyclovir resistance remains rare in immunocompetent persons, Schalkwijk et al., in their thorough analysis “Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives,” stress that it is more common in immunocompromised patients. Regardless of treatment history, the authors observe that in immunocompetent people, the frequency of acyclovir-resistant HSV is often < 1%. Higher rates are seen in immunocompromised people, especially hematopoietic stem cell transplant patients, nevertheless.
Resistance mainly arises from mutations in the DNA polymerase gene or, more usually, the viral thymidine kinase gene. These alterations may cause either lower sensitivity of the viral DNA polymerase to the active form of the medication or diminished activation of acyclovir. Resistance has major clinical consequences as, especially in immunocompromised people, it may cause either chronic or progressive infections.
Preclinical and Clinical Studies
Preclinical research of acyclovir focused on its mechanism of action and effectiveness against many herpes viruses. Its strong reduction of viral DNA synthesis in infected cells shown in vitro experiments spares uninfected host cells. Before human tests, animal models—especially in mice and guinea pigs—provided vital information on the drug’s safety and effectiveness profile.
Clinical research on the safety and effectiveness of acyclovir across many herpes virus infections have been quite thorough. Early studies found it to be better than placebo at treating shingles, cold sores, and genital herpes. Later research improved dosages and investigated its usage in other patient types, including infants and immunocompromised people.
Post-authorization Studies and Pharmacovigilance
Identification of uncommon side effects and improvement of the safety profile of acyclovir have depended much on post-marketing monitoring. Long-term observational studies have yielded important information on the drug’s safety in pregnancy and its possible ability to lower HIV acquisition risk in HSV-2 seropositive people.
Particularly in individuals with renal impairment, pharmacovigilance initiatives have raised knowledge of neurological adverse effects. As Aboelezz and Mahmoud highlight in their scoping study, acyclovir-induced neurotoxicity is uncommon but nevertheless a cause for worry particularly in cases of high dosages for diseases such viral encephalitis.
Pharmacokinetic Characteristics of Acyclovir
Acyclovir has complicated pharmacokinetics under impact of many elements, including renal function and route of administration. Because to inadequate absorption, it has low bioavailability (15–30%) when given orally. For severe infections, intravenous treatment is the recommended path as it yields better and more constant plasma concentrations.
The medication is found extensively in all bodily tissues and fluids, including cerebrospinal fluid. In adults with normal renal function, its half-life is somewhat short—2 to 3 hours—which calls for regular dosage. Renal excretion mostly removes acyclovir; tubular secretion and glomerular filtration both help to clear it.
Current Research Directions and Future Perspectives
Current research on acyclovir focuses on several key areas:
- Overcoming resistance: Developing new formulations or combination therapies to address acyclovir-resistant HSV strains.
- Improved delivery systems: Exploring novel drug delivery methods to enhance bioavailability and reduce dosing frequency.
- Expanded indications: Investigating acyclovir’s potential in treating or preventing other viral infections, including COVID-19.
- Personalized dosing strategies: Utilizing pharmacogenomics to optimize dosing regimens based on individual patient characteristics.
Future perspectives include the development of long-acting formulations and the potential for acyclovir to serve as a template for designing new antiviral agents with broader spectrum activity.
Effectiveness
It is abundantly clear that acyclovir treats herpes virus infections effectively. In many different herpes infections, including vaginal herpes, cold sores, and shingles, it greatly shortens the length and intensity of symptoms. Early start of therapy with intravenous acyclovir has greatly improved results in herpes encephalitis, lowering fatality rates from 70% to around 20% (Aboelezz and Mahmoud).
Particularly helpful for people with repeated recurrences or those receiving immunosuppressive treatment, the medication’s efficacy spans its preventive application in avoiding recurring herpes outbreaks.
Comparative Efficacy, Systematic Reviews and Meta-analyses
Comparatively to placebo, systematic reviews and meta-analyses have repeatedly shown acyclovir’s effectiveness and comparability to more modern antiviral drugs. Oral acyclovir was useful in lowering the length and intensity of initial genital herpes outbreaks, according a Cochrane study. With a comparable safety profile, another meta-analysis found that acyclovir treated herpes zoster as effectively as valacyclovir.
Comparative investigations of herpes encephalitis have confirmed acyclovir’s status as the gold standard therapy. Nonetheless, as the scoping study by Aboelezz and Mahmoud emphasizes, there are still unresolved issues about ideal dosages especially in specific groups like obese patients or those with changed renal function.
Scientific Research
Analysis of the Research Study “Acyclovir dosing in herpes encephalitis: A scoping review”
This comprehensive scoping review, conducted by Asma Aboelezz and Sherif Hanafy Mahmoud, provides a critical examination of acyclovir dosing strategies in the treatment of herpes encephalitis. Published in the Journal of the American Pharmacists Association in 2024, this study addresses a crucial gap in the literature regarding optimal acyclovir dosing, particularly in obese patients.
Methodology and Scope
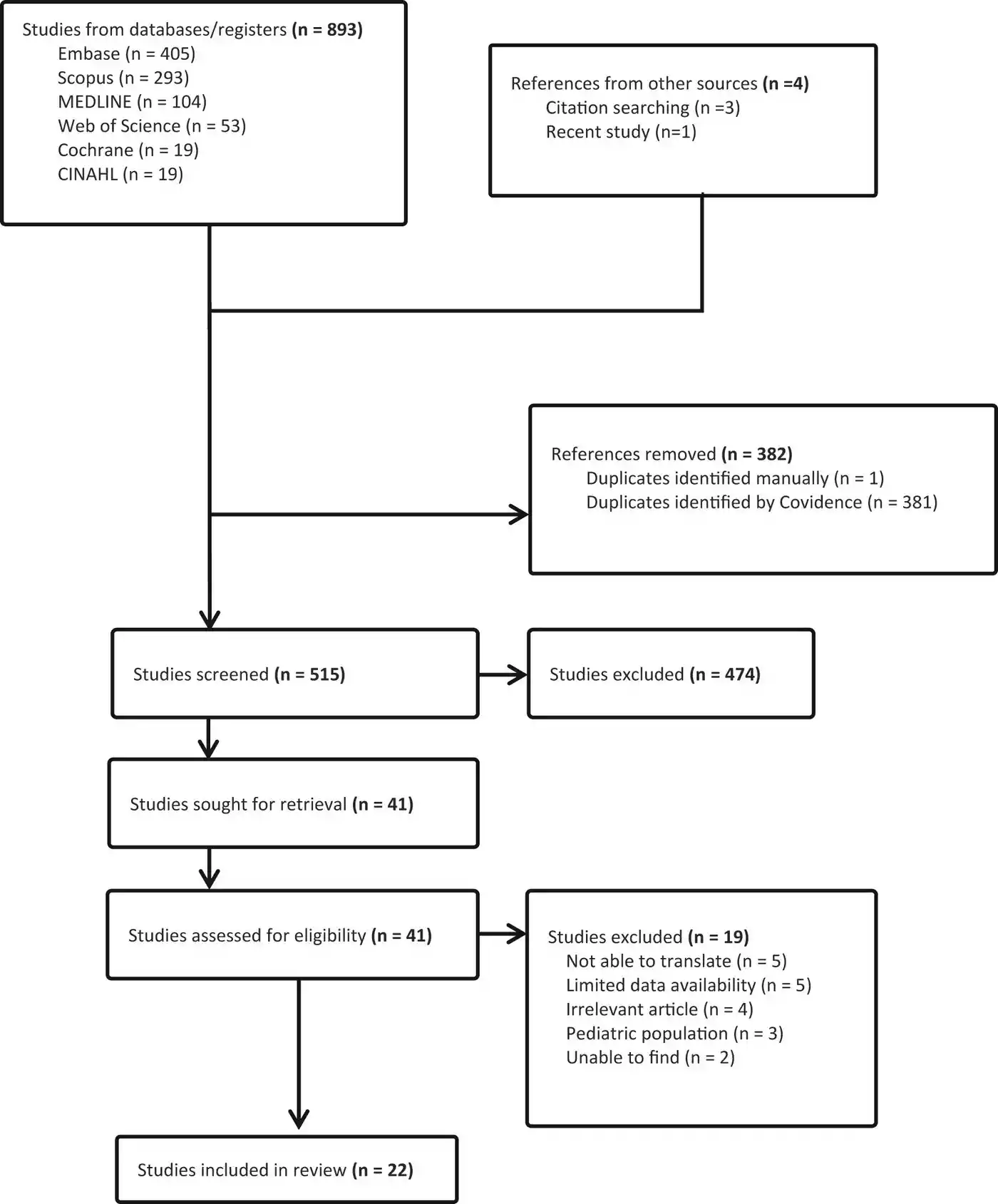
The authors employed a rigorous methodology, searching multiple databases including MEDLINE, EMBASE, Scopus, Web of Science, and CINAHL. Their search, conducted on May 26, 2023, was comprehensive and without language restrictions. The use of Covidence software for screening and selection of articles enhances the reliability of their selection process.
Key Findings
- Prevalence of Adverse Effects: The review revealed that acyclovir-associated nephrotoxicity ranged from 13% to 21%. Interestingly, the prevalence of neurotoxicity was not clearly defined, highlighting a significant gap in current research.
- Dosing Challenges in Obese Patients: A critical finding was the lack of consensus on which body weight to use for calculating acyclovir doses in obese patients. The authors note that using ideal body weight might result in subtherapeutic concentrations, while actual body weight could lead to toxicity.
- Proposed Dosing Approach: The review suggests a nuanced approach to acyclovir dosing. For patients with normal kidney function, the authors recommend using actual weight for normal-weight patients and adjusted body weight for obese patients. In cases of augmented renal clearance, they suggest doses up to the maximum recommended.
- Pharmacokinetic Considerations: The study highlights the importance of understanding acyclovir’s pharmacokinetics, especially in special populations. This includes considerations for renal function and body composition.
Strengths of the Study
- Comprehensive Database Search: The inclusion of multiple databases ensures a wide coverage of available literature.
- No Language Restrictions: This approach minimizes bias that could result from excluding non-English studies.
- Focus on a Critical Clinical Issue: The study addresses an important gap in knowledge regarding acyclovir dosing in a specific patient population.
Limitations and Future Directions
- Limited Data on Neurotoxicity: The authors note the lack of clear data on acyclovir-induced neurotoxicity, suggesting a need for further research in this area.
- Need for Comparative Studies: The review highlights the necessity for studies comparing acyclovir concentrations between obese and non-obese patients, correlating these with clinical outcomes.
Clinical Implications
This scoping review has significant implications for clinical practice. It underscores the need for personalized dosing strategies, particularly in obese patients and those with altered renal function. The authors’ proposed approach provides a framework for clinicians, but also emphasizes the need for careful monitoring and potential dose adjustments based on individual patient factors.
Conclusion and Future Perspectives
Particularly for obese individuals, Aboelezz and Mahmoud come to the conclusion that further study is absolutely necessary to provide evidence-based dose recommendations for acyclovir in herpes encephalitis. With an especially eye on linking these concentrations with clinical outcomes, they advise further research on the comparison of intravenous acyclovir concentrations between obese and non-obese individuals.
By summarizing present knowledge, pointing out important gaps, and suggesting future research possibilities, this scoping review adds much to the area. Clinicians and researchers in fields such infectious illnesses, pharmacology, and critical care medicine depend on it absolutely. The work of the writers emphasizes the complicated interaction of medication pharmacokinetics, patient traits, and clinical results in the treatment of major viral diseases including herpes encephalitis.
Briefly
Acyclovir is an antiviral medication primarily used to treat infections caused by herpes simplex viruses (HSV-1 and HSV-2) and varicella-zoster virus (VZV). It acts by preventing viral DNA synthesis, hence controlling viral replication. Prescriptions for ailments like genital herpes, cold sores, shingles, and chickenpox usually call for accyclovir. Although usually tolerated, it may have adverse effects ranging from modest gastrointestinal problems to uncommon but severe neurological consequences. Especially in specific groups like obese patients or those with renal impairment, dosage issues are rather important. Constant research keeps improving its usage and investigating possible new uses in antiviral treatment.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Aboelezz, Asma, and Sherif Hanafy Mahmoud. “Acyclovir dosing in herpes encephalitis: A scoping review.” Journal of the American Pharmacists Association, 2024, sciencedirect.com
- Schalkwijk, H. H., R. Snoeck, and G. Andrei. “Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives.” Biochemical Pharmacology, 2022, sciencedirect.com
FAQ
What are the most common acyclovir side effects?
Common acyclovir side effects include nausea, headache, and dizziness. Some patients may experience mild gastrointestinal disturbances. These effects are usually temporary and resolve on their own. Always consult your doctor if side effects persist or worsen.
How does acyclovir cream differ from oral acyclovir?
Acyclovir cream is a topical formulation applied directly to affected skin areas, primarily for cold sores. It has fewer systemic side effects compared to oral acyclovir but may be less effective for more severe infections. Consult your healthcare provider for the most appropriate form.
What is the typical acyclovir dosage for cold sores?
The standard acyclovir dosage for cold sores is 400 mg taken orally five times daily for 5 days. However, dosages can vary based on individual factors. Always follow your doctor's prescription and instructions, as they may adjust the dosage based on your specific condition.
Can I use acyclovir ointment for genital herpes?
Acyclovir ointment is primarily used for cold sores on the lips and face. For genital herpes, oral acyclovir is typically prescribed. Always consult your healthcare provider for the most appropriate treatment for genital herpes, as they will consider various factors specific to your case.
How does acyclovir compare to valacyclovir?
Acyclovir and valacyclovir are both antiviral medications used to treat herpes infections. Valacyclovir is a prodrug of acyclovir, offering better oral bioavailability. This allows for less frequent dosing compared to acyclovir. Your doctor will determine which medication is best for your specific situation.
Are there any foods to avoid while taking acyclovir?
There are no specific foods that must be strictly avoided while taking acyclovir. However, maintaining a balanced diet and staying well-hydrated can support your overall health during treatment. Always inform your healthcare provider about your diet and any supplements you're taking.
How long does it take for acyclovir to work?
Acyclovir typically starts working within 24-72 hours of beginning treatment. However, the full effect may take several days. It's crucial to start treatment as early as possible and complete the full course as prescribed by your doctor, even if symptoms improve.
Can I get acyclovir cream over the counter?
In many countries, acyclovir cream is available only by prescription. Some over-the-counter alternatives may exist, but they may not be as effective. Always consult a healthcare professional before using any medication for herpes infections to ensure proper treatment.
What is the recommended acyclovir dose for shingles?
The typical acyclovir dose for shingles is 800 mg taken orally five times daily for 7-10 days. However, dosages can vary based on individual factors and severity of the outbreak. Always follow your doctor's prescription, as they will determine the most appropriate dosage for your specific case.