Generic Name: Amitriptyline
Brand Names: Various around the world
Drug Class: Tricyclic antidepressant
Amitriptyline Side Effects, Uses, Dosage, and More
What is amitriptyline?
Amitriptyline is a tricyclic antidepressant medication used to treat depression and certain types of nerve pain. It works by increasing levels of serotonin and norepinephrine in the brain to help regulate mood and pain signaling. Amitriptyline is available as a generic drug and is marketed under various brand names by pharmaceutical companies worldwide.
Doctors may prescribe amitriptyline to treat major depressive disorder, anxiety disorders, fibromyalgia, migraines, and neuropathic pain conditions. It is sometimes used off-label at lower doses to help with insomnia. Amitriptyline is typically taken orally as a tablet.
Mechanism of action: Inhibits reuptake of serotonin and norepinephrine neurotransmitters in the brain.
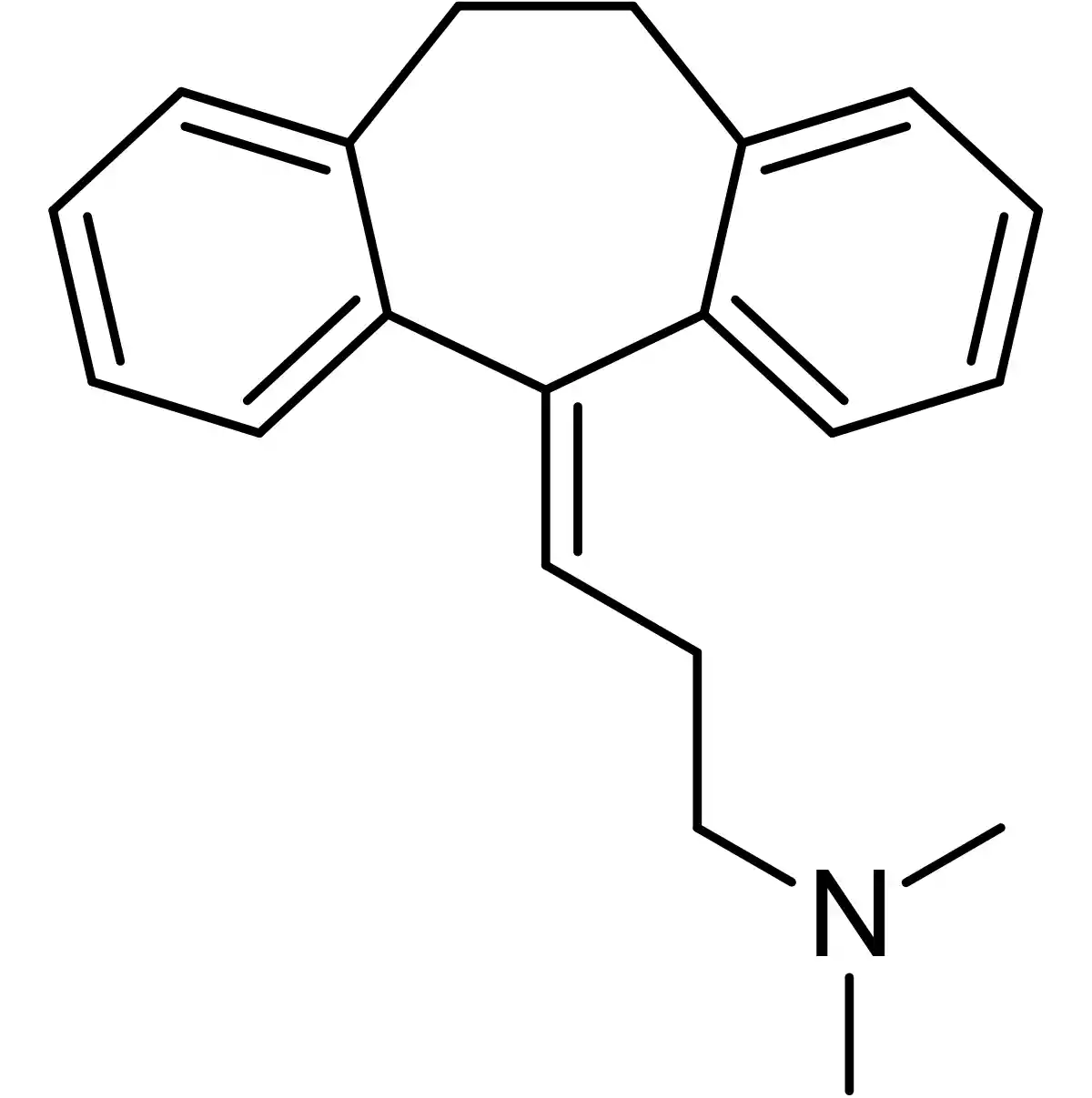
Chemical structure: Dibenzocycloheptene derivative with a side chain containing a tertiary amine.
Therapeutic category: Antidepressant, analgesic, antimigraine agent.
Anatomical/therapeutic/chemical (ATC) classification
ATC Code: N06AA09 Title: Amitriptyline Classification: Nervous system; Psychoanaleptics; Antidepressants; Non-selective monoamine reuptake inhibitors
History of Medicine
Scientists at Merck Sharp & Dohme originally synthesised amitriptyline in 1960. Approved by the FDA in 1961 for the treatment of depression, it is among the first tricyclic antidepressants ever launched into the market. Although its usage has slightly decreased with the arrival of more modern antidepressants with less adverse effects, Amitriptyline rapidly became a generally prescribed antidepressant because of its effectiveness.
In this article, we will analyze recent research on amitriptyline’s effects and applications, including studies by Bakker et al. on its off-label use for insomnia, Petkoska’s comparison of amitriptyline and mirtazapine side effects, and Farag et al.’s network meta-analysis of amitriptyline for fibromyalgia treatment. These studies provide valuable insights into the benefits and risks of amitriptyline in various clinical contexts.
Indications of amitriptyline
Amitriptyline serves as a versatile medication, primarily prescribed for:
- Major depressive disorder
- Chronic pain conditions, including fibromyalgia and neuropathic pain
- Migraine prevention
- Anxiety disorders
In recent years, off-label use for insomnia has gained attention. Bakker et al. conducted a study exploring low-dose amitriptyline for sleep maintenance problems, reporting that “73.9% of the total study population reported improvement of sleep maintenance” (Bakker et al., “Off-label low dose amitriptyline for insomnia disorder: Patient-reported outcomes”).
Contraindications and Precautions
Amitriptyline should be avoided in patients with:
- Recent myocardial infarction
- Arrhythmias
- Severe liver disease
- Known hypersensitivity to tricyclic antidepressants
- Concomitant use of monoamine oxidase inhibitors (MAOIs)
Caution is advised in patients with:
- Cardiovascular disease
- Seizure disorders
- Urinary retention
- Angle-closure glaucoma
- Hyperthyroidism
Special warnings for the elderly, children and pregnant women
Elderly: Older adults may be more sensitive to side effects, particularly anticholinergic effects and orthostatic hypotension. Lower doses are typically recommended.
Children: Amitriptyline is generally not recommended for children under 12 years old due to limited safety data.
Pregnant women: The potential risks to the fetus should be weighed against the benefits of treatment. Close monitoring is essential if used during pregnancy.
Dosage and administration
Dosage varies depending on the indication and individual patient factors. Generally:
- Depression: 75-150 mg/day, often divided into multiple doses
- Chronic pain: 10-50 mg/day, typically as a single bedtime dose
- Migraine prevention: 10-25 mg/day, usually at bedtime
Dosage should be adjusted gradually under medical supervision.
What should I do if I miss a dose?
If a dose is missed, take it as soon as remembered. However, if it’s close to the next scheduled dose, skip the missed dose and resume the regular dosing schedule. Never double up on doses to make up for a missed one.
Uses of amitriptyline
Beyond its primary indications, amitriptyline has shown promise in treating:
- Irritable bowel syndrome
- Interstitial cystitis
- Post-traumatic stress disorder
- Eating disorders
Farag et al.’s network meta-analysis highlighted amitriptyline’s efficacy in fibromyalgia, noting that it was “associated with large improvement in sleep and QoL, a moderate improvement in fatigue, a small improvement in pain” (Farag et al., “Comparison of amitriptyline and us food and drug administration–approved treatments for fibromyalgia”).
Overdose
Amitriptyline overdose can be life-threatening. Symptoms may include:
- Severe drowsiness
- Confusion
- Seizures
- Cardiac arrhythmias
- Coma
Immediate medical attention is crucial in cases of suspected overdose.
Interactions
Drug-drug interactions
Amitriptyline may interact with numerous medications, including:
- MAOIs: Can cause potentially fatal serotonin syndrome
- SSRIs: May increase risk of serotonin syndrome
- Anticholinergic drugs: Can exacerbate side effects
- CYP2D6 inhibitors: May increase amitriptyline blood levels
Drug-food interactions
- Grapefruit juice: May increase amitriptyline blood levels
- Alcohol: Can enhance CNS depression
- St. John’s Wort: May decrease amitriptyline effectiveness
Petkoska’s study on side effects noted that “patients in the mirtazapine arm in week 6 reported statistically more significant attributed SEs than those in the placebo arm”, highlighting the importance of monitoring for interactions and side effects (Petkoska, “Side effects of low-dose mirtazapine and amitriptyline in patients with insomnia”).
Amitriptyline Side Effects
Amitriptyline, while effective for various conditions, can produce a range of side effects. Bakker et al.’s study on off-label use for insomnia reported that “66.1% reported at least one side effect” (Bakker et al., “Off-label low dose amitriptyline for insomnia disorder: Patient-reported outcomes”). Common side effects include:
- Drowsiness: Often most pronounced in the initial weeks of treatment
- Dry mouth: Can lead to dental issues if persistent
- Constipation: May require dietary adjustments or laxatives
- Blurred vision: Usually temporary but can be bothersome
- Weight gain: Potentially significant and may affect treatment adherence
- Dizziness: Particularly when standing up quickly
Petkoska’s comparative study noted that amitriptyline’s side effect profile differed from other medications like mirtazapine, emphasizing the need for individualized treatment approaches (Petkoska, “Side effects of low-dose mirtazapine and amitriptyline in patients with insomnia”).
Rare but Possible Amitriptyline Side Effects
While less common, some patients may experience more serious side effects:
- Cardiac arrhythmias: Particularly in those with pre-existing heart conditions
- Seizures: Risk may increase at higher doses
- Severe allergic reactions: Including skin rashes and anaphylaxis
- Hyponatremia: Especially in older adults
- Serotonin syndrome: When combined with other serotonergic medications
Farag et al.’s meta-analysis highlighted the importance of monitoring for these rarer side effects, especially when using amitriptyline for conditions like fibromyalgia (Farag et al., “Comparison of amitriptyline and us food and drug administration–approved treatments for fibromyalgia”).
How to Manage Amitriptyline Side Effects
Managing side effects is crucial for treatment success and patient well-being. Strategies include:
- Gradual dose titration: Starting with a low dose and slowly increasing can help minimize initial side effects. As Bakker et al. noted, many patients benefit from doses as low as 10-20 mg for sleep maintenance issues.
- Timing of doses: Taking the medication at bedtime may help mitigate daytime drowsiness.
- Hydration and dietary changes: Increasing fluid intake and fiber consumption can help manage constipation.
- Regular monitoring: Especially for cardiac function and electrolyte levels in at-risk patients.
- Lifestyle adjustments: Exercise and dietary modifications may help manage weight gain.
- Oral hygiene: Regular dental check-ups and increased oral care can mitigate dry mouth effects.
- Medication adjustments: In some cases, your healthcare provider may recommend splitting doses or switching to a different formulation.
- Complementary treatments: For example, using artificial tears for dry eyes or blurred vision.
Petkoska’s research emphasized the importance of patient education and follow-up, noting that some side effects may diminish over time: “As estimated with the GEE model these differences decreased after 12 weeks of treatment” (Petkoska, “Side effects of low-dose mirtazapine and amitriptyline in patients with insomnia”).
Open communication with your doctor about any adverse effects is really vital. They may provide individualized recommendations and change course in response to need. Never change or stop your prescription schedule without expert advice as sudden stop might cause withdrawal symptoms.
Remember, even while side effects might be alarming, many patients find, with appropriate treatment, the advantages of amitriptyline exceed the negative ones. Your healthcare team can guide you toward ideal treatment results from this balance.
Additional Important Information of Amitriptyline
Resistance Development
Like other tricyclic antidepressants, amitriptyline may show declining effectiveness over time in certain people. Though not completely understood, this phenomena—often known as tachyphylaxis or tolerance—may include adaptive modifications in neurotransmitter systems. Potential resistance development should be known to clinicians, so their treatment plans should be modified.
Preclinical and Clinical Studies
Preclinical studies have elucidated amitriptyline’s complex pharmacological profile, including its effects on multiple neurotransmitter systems and ion channels. These investigations have provided insights into the medication’s diverse therapeutic applications beyond depression.
Clinical trials have explored amitriptyline’s efficacy in various conditions. Notably, Farag et al. conducted a comprehensive network meta-analysis comparing amitriptyline to FDA-approved treatments for fibromyalgia. Their findings revealed that “amitriptyline was associated with large improvement in sleep and QoL, a moderate improvement in fatigue, a small improvement in pain” (Farag et al., “Comparison of amitriptyline and us food and drug administration–approved treatments for fibromyalgia”). This study underscores the medication’s potential in managing complex chronic pain conditions.
Post-authorization studies and Pharmacovigilance
Post-marketing surveillance has been crucial in identifying rare adverse effects and long-term safety profiles of amitriptyline. Pharmacovigilance efforts have contributed to refining prescribing guidelines and identifying at-risk populations.
Bakker et al.’s study on off-label use for insomnia provided valuable real-world data, reporting that “66.1% reported at least one side effect” (Bakker et al.). This research highlights the importance of ongoing monitoring and patient education, even for well-established medications.
Pharmacokinetic characteristics of amitriptyline
Amitriptyline exhibits complex pharmacokinetics. It undergoes extensive first-pass metabolism in the liver, primarily via CYP2D6 and CYP2C19 enzymes. The active metabolite, nortriptyline, contributes significantly to its therapeutic effects. Amitriptyline’s long half-life (10-50 hours) allows for once-daily dosing in many cases.
Genetic polymorphisms in metabolizing enzymes can significantly affect drug concentrations and, consequently, efficacy and side effect profiles. This variability underscores the importance of individualized dosing strategies.
Current research directions and future perspectives
Current research focuses on optimizing amitriptyline’s use in various conditions and minimizing adverse effects. Areas of interest include:
- Personalized medicine approaches based on genetic profiling
- Novel drug delivery systems to improve tolerability
- Combination therapies to enhance efficacy and reduce side effects
- Exploration of amitriptyline’s potential in neurodegenerative disorders
Future perspectives may involve developing more selective compounds that retain amitriptyline’s beneficial effects while reducing its side effect burden.
Effectiveness
Amitriptyline’s effectiveness varies across indications. For depression, it remains a second-line treatment due to its side effect profile. However, its efficacy in pain management and sleep disorders continues to be recognized.
Petkoska’s study comparing low-dose amitriptyline and mirtazapine for insomnia provided insights into its effectiveness and tolerability in this context. The research noted that “the results of this study may help, support, and facilitate the decision-making in general practice for use of mirtazapine and amitriptyline as off-label treatment of insomnia” (Petkoska, “Side effects of low-dose mirtazapine and amitriptyline in patients with insomnia”).
Comparative efficacy, Systematic reviews and meta-analyses
Meta-analyses and systematic reviews have proved very helpful in orienting amitriptyline within the therapeutic terrain. These trials have pitted amitriptyline’s effectiveness and tolerability against those of other antidepressants, painkillers, and sleep aids.
The network meta-analysis by Farag et al. offered a thorough contrast of amitriptyline with FDA-approved fibromyalgia therapies. The results of this research confirm the function amitriptyline plays in controlling fibromyalgia symptoms, especially tiredness and disturbed sleep.
Development of guidelines and clinical decision-making depend on such comparison studies being very vital. They underline how relevant amitriptyline is still in contemporary pharmacotherapy even with more recent drugs at hand. These trials also highlight, nonetheless, the necessity of careful patient selection and monitoring to maximize results and reduce side effects.
Scientific Research
Analysis of the Research Study “Comparison of Amitriptyline and US Food and Drug Administration–Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis”
This comprehensive study, conducted by Hussein M. Farag, Ismaeel Yunusa, Hardik Goswami, Ihtisham Sultan, Joanne A. Doucette, and Tewodros Eguale, provides valuable insights into the comparative effectiveness of amitriptyline and FDA-approved treatments for fibromyalgia.
Study Design and Methodology
The researchers employed a systematic review and network meta-analysis approach, which allows for the comparison of multiple treatments simultaneously, even in the absence of direct head-to-head trials. This methodology is particularly valuable in the context of fibromyalgia, where various treatments exist but direct comparisons are limited.
Key aspects of the methodology include:
- Systematic searches of major databases (PubMed/MEDLINE, Cochrane Library, Embase, and Clinicaltrials.gov)
- Inclusion of randomized clinical trials (RCTs) comparing amitriptyline or FDA-approved doses of investigated drugs
- Use of a random-effects Bayesian network meta-analysis
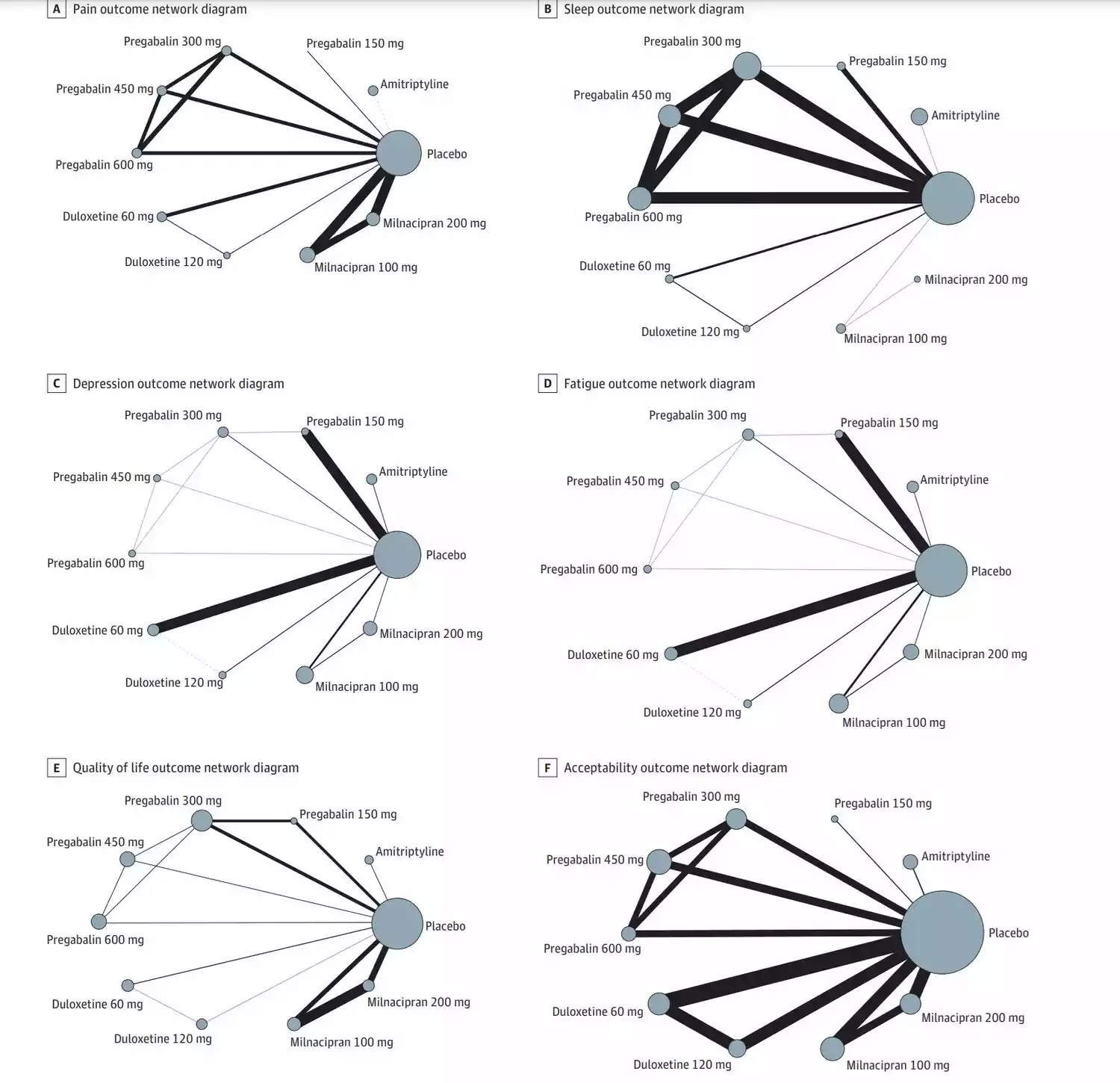
- Analysis of multiple outcomes, including pain, sleep problems, depression, fatigue, quality of life, and acceptability
Main Findings
The study included 36 RCTs with a total of 11,930 patients, providing a robust dataset for analysis. Key findings include:
- Pain management: Duloxetine 120 mg was associated with the highest pain reduction compared to placebo.
- Sleep improvement: Amitriptyline showed the greatest improvement in sleep disturbances.
- Depression: Duloxetine 120 mg and 60 mg were most effective in reducing depressive symptoms.
- Fatigue: Amitriptyline demonstrated the highest efficacy in reducing fatigue.
- Quality of life: Amitriptyline was associated with the greatest improvement in overall quality of life.
- Acceptability: Amitriptyline was the only treatment that did not show inferior acceptability compared to placebo.
Critical Analysis
Strengths:
- Comprehensive inclusion of both FDA-approved treatments and the commonly used off-label amitriptyline
- Use of advanced statistical methods (network meta-analysis) to compare treatments indirectly
- Analysis of multiple outcomes relevant to fibromyalgia management
- Large sample size, enhancing the reliability of findings
Limitations:
- Heterogeneity in study designs and outcome measures across included trials
- Potential for publication bias, although this was assessed using funnel plots
- Limited long-term data on efficacy and safety
Implications for Clinical Practice
The study’s findings suggest that treatment choice for fibromyalgia should be tailored to individual symptom profiles. For instance:
- Amitriptyline may be particularly beneficial for patients with significant sleep disturbances and fatigue
- Duloxetine could be preferred for those with more pronounced pain and depressive symptoms
The authors emphasize the importance of considering both efficacy and acceptability when making treatment decisions. They note that “clinicians should consider how treatments could be tailored to individual symptoms, weighing the benefits and acceptability, when prescribing medications to patients with fibromyalgia.”
Future Research Directions
This study highlights several areas for future research:
- Direct head-to-head comparisons of amitriptyline with FDA-approved treatments
- Long-term studies to assess the durability of treatment effects and safety profiles
- Investigations into combination therapies, given the multifaceted nature of fibromyalgia symptoms
- Exploration of personalized medicine approaches to optimize treatment selection
Conclusion
Research by Farag et al. offers a thorough picture of how well FDA-approved therapies for fibromyalgia compare to amitriptyline. Through network meta-analysis and data synthesis from many RCTs, the research provides important direction for clinical decision-making in fibromyalgia treatment. The results highlight the complicated character of fibromyalgia and the need of customized treatment plans.
This research offers a unique knowledge of the relative merits of many pharmaceutical alternatives for fibromyalgia, therefore contributing significantly to the area. Despite its off-label position, it also emphasizes how relevant amitriptyline is still for managing fibromyalgia, especially for sleep and tiredness problems. This paper lays a strong basis for further studies and clinical practice recommendations as fibromyalgia research develops.
Briefly
Amitriptyline, a tricyclic antidepressant, plays a significant role in treating various conditions beyond depression. Its mode of action is blocking brain serotonin and norepinephrine reuptake. Although mostly used for severe depressive illness, amitriptyline has demonstrated effectiveness in treating neuropathic pain and fibromyalgia among other chronic pain disorders. Its many uses include off-label usage for sleeplessness and migraine prophylaxis. Amitriptyline’s adverse effect profile and possibility for interactions with other drugs call for cautious thought even if it is very effective.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Bakker, M. H., et al. “Off‐label low dose amitriptyline for insomnia disorder: Patient‐reported outcomes.” Pharmacoepidemiology and drug safety, 2023. onlinelibrary.wiley.com
- Farag, Hussein M., et al. “Comparison of Amitriptyline and US Food and Drug Administration–Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis.” JAMA network open, 2022. jamanetwork.com
- Petkoska, I. “Side effects of low-dose mirtazapine and amitriptyline in patients with insomnia: a randomized, double-blind, placebocontrolled trial.” 2024. studenttheses.uu
FAQ
What are the common amitriptyline side effects in females?
Women taking amitriptyline may experience side effects such as weight gain, dry mouth, constipation, and changes in libido. Menstrual irregularities and breast swelling can also occur. It's crucial to discuss any concerns with your healthcare provider, as they can help manage these effects or adjust treatment if necessary.
How does amitriptyline affect blood pressure?
Amitriptyline can influence blood pressure, potentially causing orthostatic hypotension (a sudden drop when standing up). Some users may experience elevated blood pressure. Regular monitoring is essential, especially when starting treatment or adjusting dosage. Consult your doctor if you notice significant changes in your blood pressure while taking amitriptyline.
What are the specific amitriptyline side effects in the elderly?
Older adults may be more sensitive to amitriptyline's side effects. These can include confusion, dizziness, and an increased risk of falls. Urinary retention and constipation may be more pronounced. Careful dosing and monitoring are crucial for elderly patients. Always consult a healthcare professional before starting or adjusting amitriptyline treatment in older individuals.
Is weight gain a common side effect of amitriptyline?
Weight gain is a frequently reported side effect of amitriptyline. It may occur due to increased appetite or changes in metabolism. The extent of weight gain varies among individuals. If weight changes are concerning, discuss dietary strategies or alternative medications with your healthcare provider. Regular exercise and a balanced diet can help manage this side effect.
Can amitriptyline cause withdrawal effects when stopping?
Abruptly stopping amitriptyline can lead to withdrawal symptoms such as nausea, headache, and sleep disturbances. Mood changes and flu-like symptoms may also occur. It's crucial to taper off the medication gradually under medical supervision. Your doctor can create a personalized plan to minimize withdrawal effects when discontinuing amitriptyline.
How do nortriptyline and amitriptyline side effects compare?
While both are tricyclic antidepressants, their side effect profiles differ slightly. Amitriptyline tends to cause more sedation and weight gain, while nortriptyline may have fewer anticholinergic effects. Both can impact blood pressure and heart rhythm. Individual responses vary, so discuss with your doctor which medication might be more suitable for your specific situation and medical history.