
Generic Name: Carisoprodol
Brand Names: Various around the world
Drug Class: Centrally acting skeletal muscle relaxants
Carisoprodol Side Effects, Uses, Dosage, and More
What is Carisoprodol?
Carisoprodol is a prescription medication used as a short-term treatment for acute musculoskeletal pain and discomfort. It belongs to a class of drugs called centrally acting skeletal muscle relaxants. Carisoprodol works by altering communication between nerves in the brain and spinal cord to relax muscles and relieve pain and stiffness.
This medication is commonly prescribed for conditions like lower back pain, neck pain, or pain associated with sprains, strains, or other muscle injuries. It is intended for short-term use of up to 2-3 weeks. Carisoprodol is available as a generic drug and is manufactured and sold under various brand names by pharmaceutical companies worldwide.
Mechanism of action: Carisoprodol acts on the central nervous system to produce muscle relaxation, though its precise mechanism is not fully understood. It may enhance inhibitory effects in the spinal cord and reticular formation.
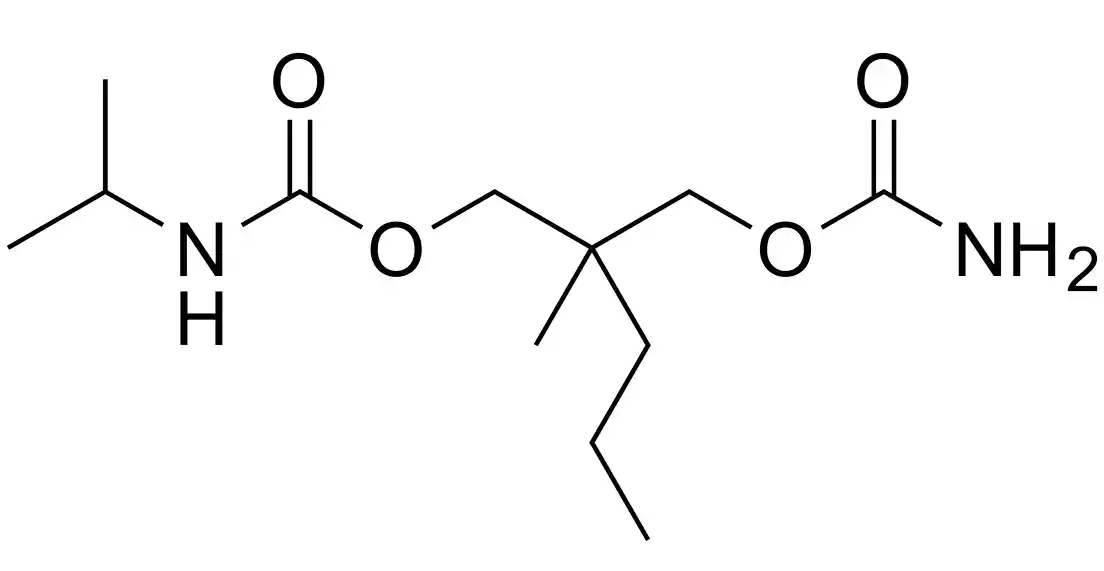
Chemical structure: Carisoprodol is an N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate.
Therapeutic category: Centrally acting skeletal muscle relaxant
Anatomical/therapeutic/chemical (ATC) classification
ATC Code: M03BA02 Title: Carisoprodol Classification: Musculo-skeletal system; Muscle relaxants; Muscle relaxants, centrally acting agents; Other centrally acting agents
History of Medicine
Carisoprodol was first synthesized in 1959 by Wallace Laboratories, a subsidiary of Carter-Wallace, Inc. It was developed as a modification of meprobamate, seeking to create a muscle relaxant with less sedative effects. The U.S. Food and Drug Administration approved carisoprodol for medical use that same year.
In this article, we will analyze several key studies on carisoprodol, including research by Calvo et al. published in the Journal of Clinical Medicine in 2022, which examined the pharmacodynamics and pharmacokinetics of carisoprodol. We will also review a study by Sai in the Annals of Musculoskeletal Medicine that investigated carisoprodol’s effects on bone health. These studies provide important insights into the medication’s mechanisms, efficacy, and potential side effects.
Indications of Carisoprodol
Carisoprodol is primarily prescribed for the short-term relief of acute musculoskeletal pain. Its main indications include:
- Acute lower back pain
- Neck pain
- Muscle spasms associated with injuries
- Pain from sprains and strains
This medication is intended for short-term use, typically not exceeding 2-3 weeks. Prolonged use may lead to dependency and is not recommended.
Contraindications and Precautions
Carisoprodol should not be used in patients with:
- Known hypersensitivity to the drug or its metabolites
- Acute intermittent porphyria
- History of substance abuse or addiction
Caution is advised in patients with:
- Liver or kidney impairment
- Seizure disorders
- Depression or suicidal thoughts
Special Warnings for the Elderly, Children and Pregnant Women
Elderly: Older adults may be more sensitive to the sedative effects of this medication. Lower doses may be necessary to prevent excessive drowsiness and falls.
Children: Safety and efficacy in pediatric patients have not been established. Use in children is generally not recommended.
Pregnant women: Carisoprodol is classified as FDA pregnancy category C. Potential risks to the fetus should be weighed against potential benefits. It should be used during pregnancy only if clearly needed.
Breastfeeding: This drug can pass into breast milk and may affect nursing infants. Consult a healthcare provider before use while breastfeeding.
Dosage and Administration
The typical adult dosage is 350 mg three times daily and at bedtime. The maximum recommended duration of use is 2-3 weeks. Dosage should be individualized based on the patient’s response and tolerance.
What Should I Do If I Miss a Dose?
If a dose is missed, it should be taken as soon as remembered. However, if it’s almost time for the next scheduled dose, skip the missed dose and resume the regular dosing schedule. Do not double up on doses to make up for a missed one.
Uses of Carisoprodol
While primarily used for musculoskeletal pain relief, some off-label uses have been reported. However, these should only be considered under strict medical supervision:
- Fibromyalgia-related pain
- Tension headaches
- Chronic pain conditions
It’s crucial to note that off-label use should be approached with caution due to the potential for dependency.
Overdose
Symptoms of overdose may include:
- Severe drowsiness
- Loss of consciousness
- Seizures
- Respiratory depression
In case of suspected overdose, immediate medical attention is crucial. Treatment is supportive and symptomatic.
Interactions
Drug-Drug Interactions
Carisoprodol can interact with various medications, potentially altering their effects or increasing side effects. Notable interactions include:
- CNS depressants (e.g., alcohol, benzodiazepines, opioids): May enhance sedative effects
- CYP2C19 inhibitors (e.g., omeprazole): May increase carisoprodol levels
- CYP2C19 inducers (e.g., rifampin): May decrease carisoprodol effectiveness
Calvo et al., in their study published in the Journal of Clinical Medicine, noted that “carisoprodol’s effect on psychomotor impairment was variable, most prominently after 1.5 h, suggesting that it is produced by carisoprodol rather than by meprobamate” (Calvo et al.). This underscores the importance of avoiding activities requiring alertness shortly after taking the medication.
Drug-Food Interactions
While specific food interactions are not well-documented, consuming alcohol while taking carisoprodol can significantly increase the risk of adverse effects and should be strictly avoided. Some patients report increased drowsiness when taking the medication with large meals, though this effect varies among individuals.
Carisoprodol Side Effects
While carisoprodol can be effective for short-term pain relief, it’s associated with various side effects. Understanding these potential adverse reactions is crucial for patients and healthcare providers alike.
Common side effects include:
- Drowsiness
- Dizziness
- Headache
- Nausea
- Vomiting
These effects typically occur within the first few hours after taking the medication. In a study published in the Journal of Clinical Medicine, Calvo et al. observed that “most significant differences were detected at 1.5 h after dosing“ regarding the drug’s effects on sedation and psychomotor impairment (Calvo et al.).
Less common but potentially more serious side effects may include:
- Confusion
- Rapid heartbeat
- Chest pain
- Difficulty breathing
- Seizures
It’s important to note that some patients may experience paradoxical reactions, such as increased anxiety or agitation, though these are relatively rare.
Rare but Possible Carisoprodol Side Effects
While less frequent, certain rare side effects warrant immediate medical attention:
- Severe allergic reactions (anaphylaxis)
- Stevens-Johnson syndrome
- Liver toxicity
- Serotonin syndrome (when combined with other serotonergic drugs)
A particularly concerning potential side effect relates to bone health. Sai, in a review published in the Annals of Musculoskeletal Medicine, highlighted that “carisoprodol may interfere with endochondral ossification, potentially leading to negative effects on bone development and health” (Sai). This finding underscores the importance of considering long-term effects, especially in patients who may require extended treatment.
Another rare but significant concern is the potential for abuse and dependence. The study by Calvo et al. noted that “no withdrawal symptoms were detected, so the risk of dependence following maximum doses and duration of treatment recommended, and under medical supervision, should be low” (Calvo et al.). However, they also emphasized the importance of adhering to recommended dosages and treatment durations.
How to Manage Carisoprodol Side Effects
Managing side effects often involves a combination of preventive measures and symptomatic treatment. Here are some strategies:
- Drowsiness and Dizziness: • Take the medication at bedtime or when you don’t need to be alert • Avoid driving or operating machinery until you know how the drug affects you
- Nausea and Vomiting: • Take the medication with food • Stay hydrated • Consider anti-nausea medications if symptoms persist
- Headache: • Ensure adequate hydration • Use over-the-counter pain relievers as directed by a healthcare provider
- Confusion or Cognitive Impairment: • Avoid activities requiring mental alertness • Report persistent symptoms to your healthcare provider
- Potential Bone Health Issues: • Discuss the need for calcium and vitamin D supplementation with your doctor • Consider regular bone density screenings if long-term use is necessary
For more severe side effects, immediate medical attention is crucial. Patients should be educated about the signs of allergic reactions, serotonin syndrome, and other serious adverse effects.
To minimize the risk of dependence, it’s essential to:
- Adhere strictly to prescribed dosages
- Limit treatment duration as recommended
- Avoid abrupt discontinuation; taper off under medical supervision
Calvo et al. emphasized that “the risk of dependence under recommended conditions of use and under strict medical supervision appears to be limited” (Calvo et al.). However, patients with a history of substance abuse should be closely monitored.
Regular follow-ups with healthcare providers are crucial for assessing the ongoing need for treatment and monitoring for potential side effects. Patients should be encouraged to report any unusual symptoms promptly.
It’s worth noting that individual responses to carisoprodol can vary significantly. What causes side effects in one patient may not affect another. Therefore, personalized management strategies, developed in consultation with healthcare providers, are often the most effective approach to mitigating and managing side effects.
Additional Important Information of Carisoprodol
Although helpful for temporary pain relief, the centrally acting muscle relaxant carisoprodol raises various issues that should be carefully discussed by patients and healthcare professionals. Its pharmacokinetic profile, possible side effects, and mechanism of action combine to create a complicated clinical picture that requires careful knowledge for best therapeutic usage.
Resistance Development
Unlike antibiotics or other antiviral drugs, carisoprodol usually does not cause the classic kind of physiological resistance. Long-term usage of tolerance to its effects, however, might cause therapeutic effectiveness to decrease gradually. This phenomena emphasizes the need of following advised temporary consumption recommendations.
To maintain the intended therapeutic impact, tolerance development could call for dosage escalation—a technique that raises the risk of side effects and reliance. Although dependence risk seems minimal under advised usage settings, the research by Calvo et al. in the Journal of Clinical Medicine underlined that there is possibility for misuse especially with longer use or greater dosages.
Preclinical and Clinical Studies
Preclinical research has shed important light on the mechanics of action and possible impacts on several physiological systems of carisoprodol. The drug’s muscle-relaxant and sedative qualities stem from animal models showing how it may control GABAergic transmission. These findings have, meantime, also sparked questions about possible long-term consequences on bone formation and condition.
Sai et al. examined preclinical data in their study in the Annals of Musculoskeletal Medicine that indicated carisoprodol could impede endochondral ossification, a vital mechanism for bone development. “Carisoprodol has been shown to reduce the activity of osteoblasts while increasing the activity of osteoclasts, leading to an imbalance in bone formation and resorption,” the writers wrote (Sai et al.). This finding emphasizes the necessity of further studies on the long-term skeletal consequences of carisoprodol, especially in populations prone for osteoporosis or other bone diseases.
Mostly, clinical trials have focused on how well carisoprodol treats acute musculoskeletal pain. Usually supporting its short-term usage, these studies have shown significant pain relief and enhanced muscular function over a placebo. But the short lifetime of most clinical research leaves mostly unresolved issues concerning long-term safety and effectiveness.
Post-authorization Studies, Pharmacovigilance and Pharmacokinetic Characteristics
Clarifying the actual safety profile of carisoprodol has been much aided by post-authorization investigations. These studies have underlined the need of following advised dosages and time rules to reduce the possibility of side effects and dependence.
Particularly when carisoprodol is taken with other central nervous system depressants, pharmacovigilance investigations have shown many areas of concern including the possibility for misuse and the danger of major adverse effects include seizures and respiratory depression. These results have spurred further government investigation and, in certain countries, reclassification of carisoprodol as a prohibited drug.
Carisoprodol’s pharmacokinetic profile greatly influences both its therapeutic activity and its side effects. Noting that “carisoprodol is rapidly absorbed from the gastrointestinal tract, with peak plasma concentrations reached within 1 to 2 hours after oral administration,” Calvo et al.’s research offered insightful analysis of the pharmacokinetics of the medication. This quick absorption matches the start of possible adverse effects and therapeutic benefits.
The medication is extensively metabolised in the liver mostly by the cytochrome P450 2C19 (CYP2C19) enzyme to produce meprobamate, its active metabolite. This metabolic route brings diversity in response depending on CYP2C19 genotype and possibility for drug-drug interactions. “Poor metabolizers showed lower carisoprodol clearance… which resulted in higher carisoprodol concentrations… which resulted in higher carisoprodol concentrations (in comparison to extensive metabolizers)” Calvo et al. noted. This result emphasizes the possible requirement of dosage changes for certain patient groups.
While its active metabolite meprobamate has a half-life of 10 hours, carisoprodol has a quite short half-life of around 2 hours. This pharmacokinetic profile helps the medicine be used in short-term pain treatment but also increases the possibility of withdrawal symptoms after sudden stop after continuous usage.
Minimizing side effects and maximizing therapeutic results depend on an awareness of these pharmacokinetic characteristics. Prescription writing for carisoprodol requires healthcare professionals to take unique patient features, concurrent drugs, and liver function into account to guarantee safe and successful therapy.
Current Research Directions and Future Perspectives
The terrain of carisoprodol research is changing as new studies concentrate on various important topics. One important focus is on possible long-term consequences on bone growth and condition. Sai’s study in the Annals of Musculoskeletal Medicine has raised questions about the influence of carisoprodol on endochondral ossification, which has spurred further research on how it affects bone metabolism (Sai). Advanced imaging methods and biomarketer analysis might be used in future research to clarify how the medicine affects bone microarchitecture and turnover.
Developing alternate formulations or delivery techniques to minimize side effects while preserving therapeutic effectiveness is another area of increasing attention. Extended-release formulations under investigation by researchers might help to lower peak-related adverse effects and perhaps lower dose frequency. Furthermore under active research are topical treatments that could provide localized muscular relaxation with low systemic dosage.
Another area of unexplored territory in study is the part genetic elements play in carisoprodol metabolism and response variability. Building on the results of Calvo et al. on CYP2C19 polymorphisms, further research may use pharmacogenomic techniques to detect other genetic markers influencing medication response and side effect vulnerability (Calvo et al.). More customized prescription practices might be made possible by this area of research, therefore maximizing treatment results and reducing dangers.
Additionally clarifying the central nervous system mechanisms of action for carisoprodol are neuroimaging investigations. Modern methods include positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) might expose the effects of the medicine on particular brain areas and neurotransmitter systems, therefore clarifying possible new therapeutic uses or helping to explain noted negative effects.
Effectiveness
Many clinical trials and real-world research have clearly shown how effectively carisoprodol controls acute musculoskeletal pain. For temporary therapy, it is a good choice because of its fast starting of action and capacity to provide both muscle relaxation and pain relief. The evaluation of efficiency, thus, has to be weighed against possible hazards and negative consequences.
With regard to sedation and psychomotor impairment, Calvo et al.’s research in the Journal of Clinical Medicine offered insightful analysis of the pharmacodynamics of carisoprodol finding that “most significant differences were detected at 1.5 h after dosing”. This quick start of action adds to the drug’s efficacy in offering quick relief but also emphasizes the need of advising patients on possible impairment in tasks requiring attentiveness.
When treating acute low back pain and other musculoskeletal disorders marked by muscular spasm, carisoprodol seems to be most effective. Its usage in chronic pain disorders is still under discussion, nevertheless, with questions over long-term safety and the possibility of dependency restricting its use in such situations.
Comparative Efficacy, Systematic Reviews and Meta-analyses
Comparative studies have aimed to place carisoprodol in the larger panorama of muscle relaxants and painkillers. Although direct head-to- head comparisons are rare, existing data indicate that for short-term usage carisoprodol’s effectiveness is equivalent to that of other often prescribed muscle relaxants such cyclobenzaprine and metroxalone.
Meta-analyses and systematic reviews have given a more whole picture of carisoprodol’s use in treatment. These studies mostly confirm the drug’s efficacy in acute musculoskeletal disorders but stress the necessity of careful usage considering the possibility of side effects and addiction. Many of these studies have a quite limited period of included data, which restricts conclusions on long-term effectiveness and safety.
Particularly in light of non-pharmacological treatments for musculoskeletal pain, one systematic review underlined the dearth of high-quality, long-term trials on carisoprodol. This disparity in the literature emphasizes the necessity of additional strong, long-term studies to guide treatment decisions.
Particularly in clarifying the pharmacokinetic and pharmacodynamic characteristics of carisoprodol, Calvo et al.’s study adds important data to this corpus of information. Their conclusion that “no withdrawal symptoms were detected, so the risk of dependence following maximum doses and duration of treatment recommended, and under medical supervision, should be low” gives comfort about short-term use but does not absolve the need of caution in prescription writing (Calvo et al.).
Meta-analyses have also looked at the relative risk-benefit ratios among many muscle relaxants, including carisoprodol. Although carisoprodol is useful, these studies usually find that its potential for misuse and sedation makes it less preferred as a first-line choice than alternatives with more mild side effect profiles.
Comparative position of carisoprodol has also been altered by changing legal environment around it, including reclassification as a prohibited drug in several countries. Future meta-analyses and systematic reviews will have to take into account these legislative changes and how they affect patient outcomes and prescription practices.
In summary, even while carisoprodol is still a good choice for temporary relief of acute musculoskeletal pain, continuous study helps us to better grasp its ideal usage, possible hazards, and long-term consequences. Future rules and informed individualized treatment strategies will depend much on the combination of pharmacokinetic, pharmacodynamic, and clinical study results.
Analysis of the Research Study “Single and Multiple Dose PK–PD Characterization for Carisoprodol. Part I: Pharmacokinetics, Metabolites, and 2C19 Phenotype Influence. Double-Blind, Placebo-Controlled Clinical Trial in Healthy Volunteers”
This comprehensive study, conducted by Aitana Calvo, Saioa Alonso, Esther Prieto, Ana Ascaso-del-Rio, Jordi Ortuño, Nieves Fernandez, and Antonio Portolés, provides crucial insights into the pharmacokinetics and pharmacodynamics of carisoprodol. Published in the Journal of Clinical Medicine in 2022, this research addresses significant gaps in our understanding of this widely prescribed muscle relaxant.
Study Design and Methodology
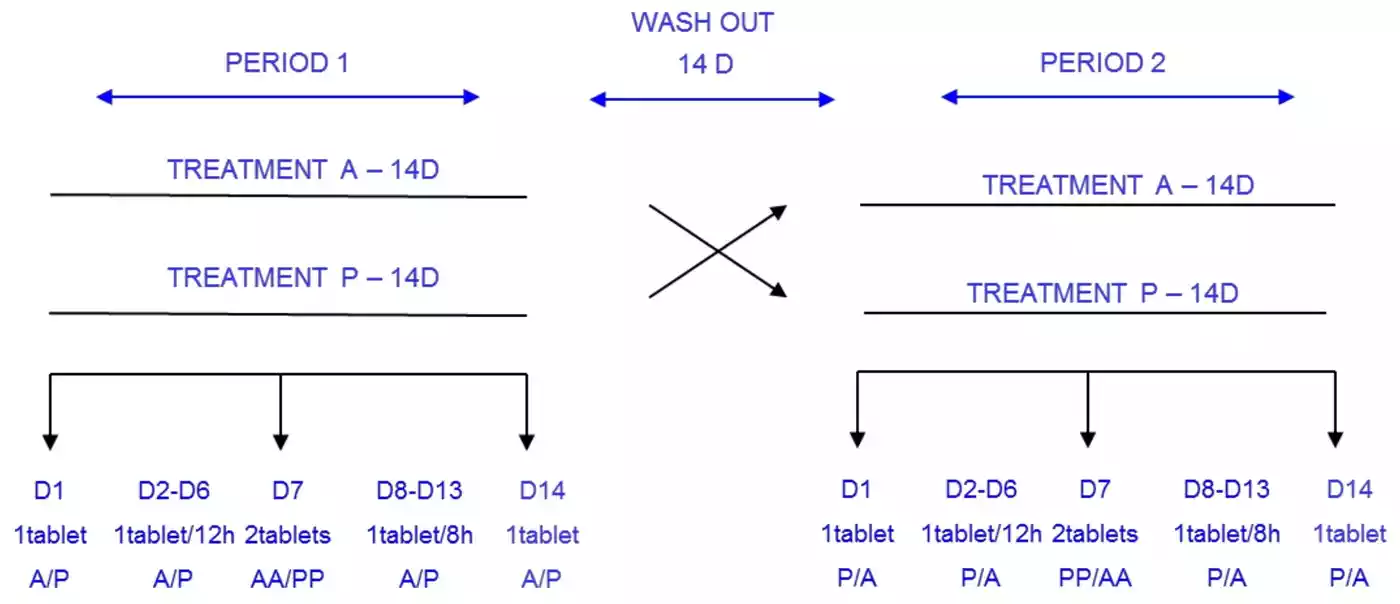
Participants and Protocol
The study employed a rigorous double-blind, placebo-controlled, randomized, crossover design involving 13 healthy volunteers. This approach minimizes bias and enhances the reliability of the results. The researchers administered carisoprodol in various dosing regimens:
- Single dose (350 mg)
- Multiple doses (350 mg/8 h for 14 days)
- Double dose (700 mg)
This comprehensive dosing strategy allows for a thorough examination of the drug’s behavior under different clinical scenarios.
Pharmacokinetic Analysis
The researchers utilized advanced analytical techniques, including high-performance liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS), to measure plasma concentrations of carisoprodol and its metabolite, meprobamate. This method ensures high sensitivity and specificity in quantifying drug levels.
Key Findings
Single Dose Pharmacokinetics
After a single 350 mg dose, the study reported:
- Mean Cmax: 2580 ± 1214 ng/mL
- Mean AUC0–∞: 8072 ± 6303 h·ng/mL
- Mean half-life: 2 ± 0.8 hours
These parameters provide a clear picture of carisoprodol’s rapid absorption and elimination.
Multiple Dose Pharmacokinetics
The 14-day multiple dose regimen revealed:
- Similar carisoprodol parameters to single-dose administration
- Accumulation of meprobamate (the active metabolite)
- Steady-state reached by day 14
This finding is particularly significant as it demonstrates the potential for metabolite accumulation with prolonged use, which could influence both efficacy and side effect profiles.
CYP2C19 Phenotype Influence
A notable aspect of this study was the examination of CYP2C19 phenotype’s impact on carisoprodol metabolism. The researchers found:
- Poor metabolizers exhibited lower carisoprodol clearance
- Higher carisoprodol concentrations in poor metabolizers
- Consequent higher meprobamate concentrations in extensive metabolizers
This genetic variation in drug metabolism underscores the potential need for personalized dosing strategies.
Critical Analysis
Strengths
- Comprehensive Design: The study’s multi-faceted approach, examining single, multiple, and double doses, provides a holistic view of carisoprodol’s pharmacokinetics.
- Genetic Considerations: The inclusion of CYP2C19 phenotype analysis adds a valuable dimension to understanding individual variability in drug response.
- Advanced Analytical Methods: The use of LC/MS/MS ensures high-quality, reliable pharmacokinetic data.
Limitations
- Sample Size: With only 13 participants, the study’s statistical power may be limited, particularly for subgroup analyses.
- Population Homogeneity: The focus on healthy volunteers may not fully represent the diverse patient population that typically receives carisoprodol.
- Duration: While the 14-day multiple dose period provides valuable data, it may not capture very long-term effects or rare adverse events.
Implications and Future Directions
This study significantly advances our understanding of carisoprodol’s pharmacokinetics and highlights several important considerations for clinical practice:
- Personalized Medicine: The observed impact of CYP2C19 phenotype suggests potential benefits in genotype-guided dosing strategies.
- Metabolite Accumulation: The accumulation of meprobamate with multiple dosing emphasizes the need for careful monitoring in long-term use scenarios.
- Dosing Regimens: The similar pharmacokinetics between single and multiple doses of carisoprodol supports current short-term use recommendations.
Future research building on this study might explore:
- Larger, more diverse patient populations
- Longer-term pharmacokinetic profiles
- Correlation of pharmacokinetic parameters with clinical outcomes and adverse events
Conclusion
The research by Calvo et al. provides a solid foundation for understanding carisoprodol’s behavior in the human body. By elucidating its pharmacokinetic profile and the influence of genetic factors, this study contributes valuable information to guide clinical decision-making and future research directions in the use of this widely prescribed muscle relaxant.
Briefly
Carisoprodol is a centrally-acting skeletal muscle relaxant prescribed for short-term relief of acute musculoskeletal pain. It produces muscular relaxation and sedative effects via adjusting central nervous system neurotransmission. Although it works well for acute problems, its usage should be carefully considered given its adverse effects and dependency risk. Recent studies have clarified its pharmacokinetics and the effect of genetic elements on metabolism as well as the buildup of meprobamate, an active metabolite, with chronic usage. These results highlight the need of tailored dosage plans and following advised treatment lengths.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Calvo, A., et al. “Carisoprodol Single and Multiple Dose PK-PD. Part II: Pharmacodynamics Evaluation Method for Central Muscle Relaxants. Double-Blind Placebo-Controlled Clinical Trial in Healthy Volunteers.” Journal of Clinical Medicine, vol. 11, no. 4, 2022, p. 1141. www.mdpi.com
- Calvo, A., et al. “Single and Multiple Dose PK–PD Characterization for Carisoprodol. Part I: Pharmacokinetics, Metabolites, and 2C19 Phenotype Influence. Double-Blind, Placebo-Controlled Clinical Trial in Healthy Volunteers.” Journal of Clinical Medicine, vol. 11, no. 3, 2022, p. 858. www.mdpi.com
- Sai, Y. “The effects of carisoprodol on endochondral ossification: A review of the literature and implications for bone health.” Annals of Musculoskeletal Medicine, vol. 6, no. 1, 2022, pp. 001-004. www.organscigroup.us
FAQ
What are the common carisoprodol side effects?
Common side effects include drowsiness, dizziness, and headache. Some patients may experience nausea, vomiting, or mild skin reactions. Serious side effects, though rare, can include allergic reactions or seizures. It's crucial to consult a healthcare provider if any side effects persist or worsen.
How does carisoprodol work in the body?
Carisoprodol's mechanism of action involves modulating neurotransmission in the central nervous system. It enhances GABA activity, leading to muscle relaxation and pain relief. The drug also metabolizes into meprobamate, which contributes to its effects. Always consult a doctor for personalized medical advice.
What drug class does carisoprodol belong to?
Carisoprodol belongs to the drug class of centrally acting skeletal muscle relaxants. It's primarily used for short-term relief of acute musculoskeletal pain and associated muscle spasms. As with any medication, its use should be under the guidance of a healthcare professional.
How does carisoprodol compare to tramadol for pain relief?
While both are used for pain relief, they work differently. Carisoprodol is a muscle relaxant, while tramadol is an opioid analgesic. Carisoprodol targets muscle spasms, whereas tramadol affects pain perception. The choice between them depends on the specific condition and should be made by a healthcare provider.
Is carisoprodol a controlled substance?
Yes, carisoprodol is classified as a Schedule IV controlled substance in the United States due to its potential for abuse and dependence. This classification imposes stricter regulations on its prescription and use. Always follow your doctor's instructions and never share this medication with others.
What is the recommended carisoprodol dosage?
The typical adult dosage is 350 mg three times daily and at bedtime. However, dosage can vary based on individual factors and the specific condition being treated. It's crucial to follow the prescribed dosage and not exceed the recommended duration of use. Always consult your healthcare provider for personalized dosing instructions.
What are important carisoprodol warnings to be aware of?
Key warnings include the potential for drowsiness, impaired driving ability, and increased risk of falls, especially in older adults. Carisoprodol can be habit-forming and should not be used long-term. It may interact with alcohol and other CNS depressants. Always inform your doctor about all medications you're taking.


