Generic Name: Celecoxib
Brand Names: Various around the world
Drug Class: Nonsteroidal anti-inflammatory drug (NSAID), selective COX-2 inhibitor
Celecoxib Side Effects, Uses, Dosage, and More
What is Celecoxib? – Chemical Structure and Mechanism of Action
Celecoxib is a nonsteroidal anti-inflammatory drug (NSAID) that selectively inhibits cyclooxygenase-2 (COX-2). It is used to treat pain and inflammation associated with various conditions, including osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute pain, and primary dysmenorrhea. Celecoxib works by reducing prostaglandin production, which are chemicals involved in inflammation.
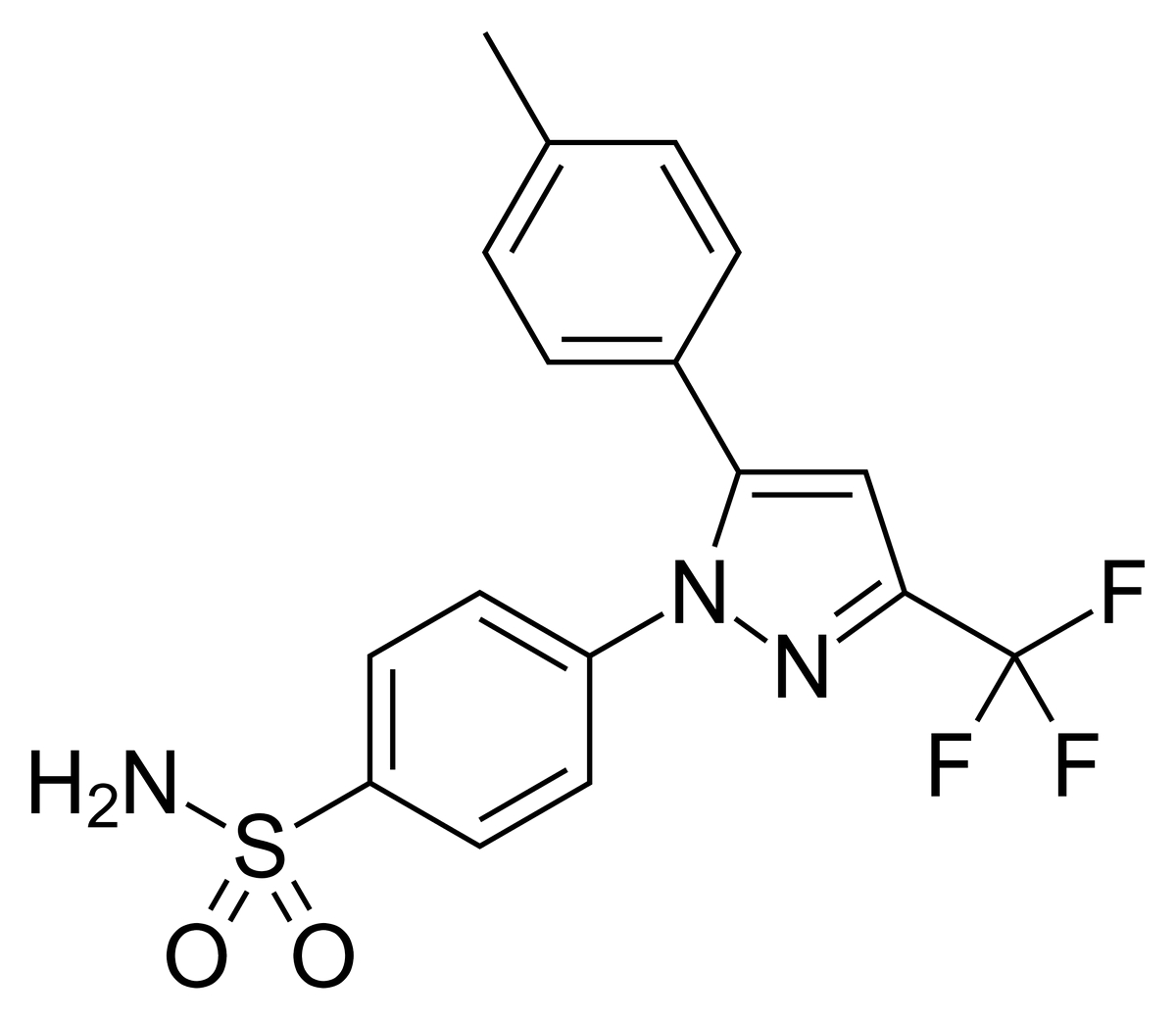
Chemically, celecoxib is 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide. It has a molecular formula of C17H14F3N3O2S and a molecular weight of 381.38 g/mol. The presence of a sulfonamide moiety in its structure defines its COX-2 selectivity and anti-inflammatory activity.
Celecoxib is available under various brand names worldwide, as many pharmaceutical companies manufacture and market this drug. It belongs to the therapeutic class of anti-inflammatory and antirheumatic products. By selectively inhibiting COX-2, celecoxib reduces inflammation while potentially causing fewer gastrointestinal side effects compared to traditional NSAIDs that inhibit both COX-1 and COX-2.
History of Medicine
Celecoxib was discovered and developed by G.D. Searle & Company and co-promoted by Pfizer under the brand name Celebrex. It was approved by the U.S. Food and Drug Administration (FDA) in December 1998, becoming the first selective COX-2 inhibitor on the market. This approval marked a significant advancement in pain management, offering a potentially safer alternative to traditional NSAIDs for patients at risk of gastrointestinal complications.
Recent research has explored new potential applications for celecoxib beyond its primary use as an anti-inflammatory agent. Studies have investigated its efficacy in mood disorders, antimicrobial activity, and cancer treatment, highlighting the ongoing interest in repurposing this well-established drug for novel therapeutic applications.
Celecoxib’s uses
Mostly acting as an anti-inflammatory and analgesic, Celecoxib is Its principal uses are in controlling inflammation and discomfort related to ankylosing spondylitis, rheumatoid arthritis, and osteoarthritis. Healthcare professionals could also recommend it to reduce primary dysmenorrhea symptoms and for relief from acute discomfort.
Potential novel uses for this medication have lately attracted attention in research. In their paper “Challenges and opportunities for celecoxib repurposing,” Bąk and Krupa underline its possible use in cancer treatment. For cancer patients suffering in depression and persistent pain, they observe that “celecoxib is a good therapeutic option for reducing pain, as well as improving mood”. In oncology, this double action makes it very useful.
Fascinatingly, Gędek et al.’s methodical study ” Celecoxib for Mood Disorders” turned up data supporting its effectiveness as an additional therapy in manic and major depression. Significant therapeutic advantages were shown by “celecoxib at a dosage of 400 mg/day used for 6 weeks as an add-on treatment in major depression and mania,” they said. This implies a possible use for the medication in psychiatric therapy, especially for people with mood disorders refractory to traditional therapies.
Moreover, new studies point to probable antibacterial qualities of celecoxib. In their work on “celecoxib-loaded cubosomal nanoparticles,” Alshawwa et al. showed in vivo efficacy against Staphylococcus aureus infections. This surprising result offers fresh opportunities for medication reuse in the control of infectious diseases.
Contraindications and Avoidance
Patients with known susceptibility to sulfonamides or NSAIDs should avoid Celecoxib. Because of the potential of severe allergic responses, those with a history of asthma, urticaria, or allergy-type reactions after aspirin or another NSAID should avoid celecoxib.
Celecoxib should not be used by patients with peptic ulcer disease or active gastrointestinal bleeding. Those with extensive renal illness or serious liver damage also need much thought before beginning therapy.
Celecoxib’s cardiovascular concerns call for prudence. Before starting therapy, patients who have a history of cardiovascular events or those who are very susceptible for such occurrences should see their doctor. The medication could raise a person’s chance of major cardiovascular thrombotic events, myocardial infarction, and stroke.
Particularly advised for the elderly, children, and pregnant women are special warnings.
Older people might be more sensitive to side effects, including renal damage and gastrointestinal problems. In this demographic, careful observation and maybe reduced doses are advised.
Children under two years old’s safety and effectiveness from celecoxib are still unknown. Dosing should be precisely changed for older children depending on body weight and the particular ailment under treatment.
Because of possible hazards to the fetus including premature closure of the ductus arteriosus and oligohydramnios, pregnant women—especially in the third trimester—should avoid celecoxib. Women who want to get pregnant should also take into account the possibility of the medication lowering female fertility.
Nursing women should be careful as celecoxib might be eliminated in breast milk. The advantages of therapy for the mother should balance any possible hazards to the newborn.
Doses and Method of Administration
Different conditions being treated and personal patient characteristics affect the dosage of celecoxib. Usually advised dosage for osteoarthritis is either 200 mg once day or 100 mg twice daily. Generally speaking, rheumatoid arthritis calls for 100–200 mg twice day.
Standard dosage for ankylosing spondylitis is either 200 mg once day or 100 mg twice daily. Often advised in situations of acute discomfort or primary dysmenorrhea is an initial dosage of 400 mg followed by an extra 200 mg if necessary on the first day, then 200 mg twice day as needed.
To reduce the risk of side effects, use the lowest effective dosage for the shortest length of time. Celecoxib should be taken by patients with meals to enhance absorption and maybe lower gastrointestinal side effects.
In their work on “Dual-targeting celecoxib nanoparticles,” Chen et al. propose that new delivery techniques might improve the effectiveness of the medicine while maybe lowering adverse effects. “celecoxib-loaded dual-targeted nanoparticle (Cel@HVGB) exhibited a sustained release profile with ROS responsiveness,” they say, which might lead to better treatment results in disorders like ulcerative colitis.
What should I do should I miss a dose?
Should a dosage be missed, it should be given right away upon memory recall. If it is near the time for the next planned dosage, however, omit the missed dose and start the usual dosing pattern once again. Patients should not double up on dosages to offset a missed one as this raises their chance of negative effects.
Maintaining therapeutic levels of the drug depends on consistency in scheduling and following the advised program. If a patient often misses doses or worries about their dosage regimen, they should see their healthcare practitioner.
Overdose
Drowsiness, stomach discomfort, nausea, vomiting, and in extreme situations gastrointestinal bleeding may all be indications of a Celecoxib overdose. Extreme circumstances may bring hypertension, acute renal failure, and respiratory depression. No particular antidote exists for a celecoxib overdose. Treatment mostly consists on supportive care: activated charcoal, gastric lavage if done shortly after consumption, and close vital sign and organ function monitoring. The great protein binding of the medication makes hemodialysis typically useless.
Interactions
Drug-drug interactions
Celecoxib may interact with many drugs, thereby changing their effectiveness or raising their side effect risk. Notable encounters comprise:
Anticoagulants: Using warfarin concurrently increases bleeding risk. One must check INRs closely.
Antihypertensives: The medicine could lower diuretics’ and ACE inhibitors’ efficacy. One should keep strict attention on blood pressure.
Aspirin: Combining drugs might raise bleeding risk and gastrointestinal ulcers.
Fluconazole: Possibly necessitating dosage changes, this antifungal may raise celecoxib plasma concentrations.
Lithium: Celecoxib may raise lithium plasma levels, which calls for cautious patient monitoring on lithium treatment.
Fascinatingly, Gędek et al. found that when taken with several antidepressants, “celecoxib did not elevate antidepressant levels” indicating possible safe co-administration in mood disorder therapies.
Drug-food interactions
Although celecoxib may be given either with or without food, high-fat meals could slow down its absorption. Given that grapefruit juice raises the plasma concentrations of the medication, one should avoid it. Because of higher gastrointestinal bleeding risk, alcohol intake should be restricted while under therapy.
Celecoxib Side Effects
Typical adverse effects include headache, dizziness, sleeplessness, edema, nausea, dyspepsia, diarrhea. In individuals with mood disorders, “celecoxib at a dosage of 400 mg/d used for up to 12 weeks appeared to be a safe treatment,” Bąk and Krupa said. They underlined, therefore, the necessity of long-term research to assess safety in recurring mood disorders.
Rare but Possible Celecoxib Side Effects
Less frequent yet quite severe negative effects might be:
Cardiovascular events include stroke and myocardial infarction
Gastrointestinal perforation, ulceration, or bleeding in general
Reactions in anaphylaxis
Hepatotoxicity
Renal failure
Toxic epidermal necrolyzed Stevens-Johnson syndrome
“celecoxib cubosomal NPs resulted in a considerable decrease (p < 0.05) in the inflammatory markers, including IL-1β, IL-6, COX-2, and TNF-α compared with celecoxib-free drugs,” Alshawwa et colleagues. noted. This implies that despite improving medicinal effectiveness, new delivery strategies might help reduce certain adverse effects.
How to Manage Celecoxib Side Effects
Managing side effects typically requires:
Dose modification: Reducing the dosage could help certain adverse effects be avoided without sacrificing effectiveness.
Timing of administration: Using meals to take the medication may assist to lower gastrointestinal adverse effects.
Especially with long-term usage, regular check-ups to evaluate hepatic, renal, and cardiovascular functions are very vital.
Combining therapy: Sometimes adding a proton pump inhibitor helps guard against gastrointestinal problems.
alternate formulations: Chen et al. found that “celecoxib-loaded dual-targeted nanoparticle (Cel@HVGB) exhibited excellent cell-specific targeting ability to both inflamed epithelium and M1 macrophages.” Future side effect profiles could be better thanks to such focused delivery strategies.
Patients should be advised to report any unexpected symptoms right away and informed of possible side effects. Should serious adverse effects arise, stopping the medication under medical care might be required. Every patient should give much thought to the harmony between possible hazards and therapeutic advantages.
Additional Important Information of Celecoxib
Resistance Development
Unlike antibiotics, celecoxib usually results in no bacterial resistance. Long-term usage of it, however, can cause adaptive reactions within the body. Tachyphylaxis, the phenomena wherein effectiveness decreases with time, might result from constant treatment. This adaptation is a physiological change to the drug’s presence rather than resistance in the conventional sense.
Some research have looked at how well celecoxib could fight antibiotic resistance. Alshawwa et al. looked explored cubosomal nanoparticles loaded with celecoxib as a fresh way to treat Staphylococcus aureus infections. Their results imply that this formulation might improve the antibacterial qualities of the medication, therefore providing a novel approach against resistant strains (Alshawwa et al.).
Preclinical and Clinical Studies
Beyond its main usage as an anti-inflammatory medication, preclinical research have extensively looked into the mechanisms of action and other uses for celecoxib. Reducing inflammation and pain in a variety of diseases, including arthritis and cancer-related pain, animal models have shown great success.
Recent studies on celecoxib’s possible use in mood disorders have looked at On celecoxib for mood disorders, Gędek et al. systematically reviewed and meta-analyzed randomized controlled trials. When administered as a supplementary therapy for serious depression and mania, they discovered evidence confirming its antidepressant effectiveness. Significant therapeutic improvements were shown by “celecoxib at a dose of 400 mg/day used for 6 weeks as an add-on treatment in major depression and mania,” the scientists reported (Gędek et al.).
Additionally looking into celecoxib’s possibilities in cancer prevention and therapy are clinical investigations. Particularly in high-risk patients, certain studies on colorectal cancer prevention indicate encouraging outcomes. Still debatable, however, these results call for further research.
Post-authorization Studies, Pharmacovigilance and Pharmacokinetic Characteristics
Monitoring Celecoxib’s long-term safety profile has been much aided by post-authorization research. These investigations have produced important information on possible medication interactions and unusual side effects that may not have been clear from first clinical trials.
Particularly in response to concerns expressed about other COX-2 inhibitors, pharmacovigilance initiatives have concentrated on tracking cardiovascular risks linked with celecoxib usage. Generally speaking, long-term safety investigations have shown that celecoxib, at advised dosages, has a similar cardiovascular risk profile to conventional NSAIDs.
With regard to pharmacokinetic properties, celecoxib is mostly metabolised in the liver by CYP2C9 and has great protein binding—about 97%. Its weak water solubility causes somewhat low bioavailability, between 20 and 40%. This has spurred studies into new formulations meant to improve its bioavailability and effectiveness.
Chen et al investigated a novel strategy to raise the therapeutic effectiveness of celecoxib in ulcerative colitis. Promising in both preserving intestinal epithelium and controlling macrophage polarization, they created a dual-targeting nanoparticle system Suggesting possible enhancements in drug delivery and effectiveness, the scientists said “celecoxib-loaded dual-targeted nanoparticle (Cel@HVGB) exhibited a sustained release profile with ROS responsiveness.”
The assessment on celecoxib repurposing by Bąk and Krupa underlined its possibilities in many therapeutic spheres beyond its main uses. To maximize celecoxib’s pharmacokinetic characteristics and reduce side effects, they spoke about possibilities and problems in creating novel formulations and delivery methods (Bąk and Krupa).
These continuous research projects and developments in drug delivery technologies help us to better grasp the pharmacological characteristics and possible therapeutic uses of celecoxib. Research advances might result in more focused and successful usage of celecoxib in different medical disorders, maybe with lower side effects and better patient results.
Effectiveness, Current Research Directions and Future Perspectives
Clearly documented is how effectively celecoxib controls inflammation and discomfort related to osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. Recent studies, however, have broad the field of possible uses by investigating its effectiveness in several therapeutic domains.
Using celecoxib to treat mood disorders is one exciting line of research. Published in the Journal of Clinical Medicine, Gędek et al., in their systematic review and meta-analysis, found evidence supporting the antidepressant effectiveness of celecoxib as an additional therapy in major depression and mania. Significant therapeutic effects were shown, according to the authors, “celecoxib at a dose of 400 mg/day used for 6 weeks as an add-on treatment in major depression and mania”. This result offers fresh opportunities for controlling mood disorders resistant to therapy.
Modern research is also concentrating on creative drug delivery techniques to maximize the therapeutic effectiveness of celecoxib and reduce side effects. Chen et al. investigated a dual-targeting nanoparticle approach for ulcerative colitis therapy with celecoxib. Their work, which was written up in the Chemical Engineering Journal, showed that this new formulation may control macrophage polarization and protect intestinal epithelium. The researchers pointed out that “celecoxib-loaded dual-targeted nanoparticle (Cel@HVGB) exhibited a sustained release profile with ROS responsiveness,” implying possible enhancements in drug delivery and effectiveness for inflammatory bowel illnesses (Chen et al.).
The possible antibacterial action of celecoxib is another area of developing inquiry. Alshawwa et al. looked explored cubosomal nanoparticles loaded with celecoxib as a therapy for Staphylococcus aureus infections. Published in Microorganisms, their results imply that this formulation may improve the antibacterial qualities of the medicine, therefore providing a novel approach against resistant strains (Alshawwa et al.).
As Bąk and Krupa’s assessment in Pharmaceutical Research emphasizes, future viewpoints for celecoxib research include more investigation of its repurposing potential. To maximize celecoxib’s pharmacokinetic characteristics and reduce side effects, the authors examined possibilities and difficulties in creating alternative formulations and delivery methods (Bąk and Krupa).
Comparative Efficacy, Systematic Reviews and Meta-analyses
With a maybe more favorable gastrointestinal safety profile, comparative effectiveness trials have typically showed celecoxib to be equally efficient as conventional NSAIDs in controlling pain and inflammation. Still up for contention and investigation, however, is whether celecoxib’s cardiovascular safety is better than that of other NSAIDs.
The PRECISION study, which matched the cardiovascular safety of celecoxib with naproxen and ibuprofen in individuals with osteoarthritis or rheumatoid arthritis, makes a major addition to this discipline. With respect to cardiovascular safety, the research revealed that celecoxib was not inferior than naproxen nor ibuprofen.
Meta-analyses and systematic reviews have given important new perspectives on the safety and effectiveness of celecoxib for many indications. The study of celecoxib for mood disorders by Gędek et al. offers a thorough examination of its possible psychiatric uses. The writers came to the conclusion that while celecoxib showed promise in manic and severe depression, data on its effectiveness in bipolar depression was equivocal.
Within the framework of inflammatory bowel illnesses, systematic reviews have shown possible advantages of celecoxib in terms of symptom control and inflammation lowering. These results, meantime, are tempered by worries regarding long-term safety, especially in individuals with a history of gastrointestinal problems.
Meta-analyses looking at how celecoxib could prevent cancer have shown conflicting findings. Although some research point to a possible preventive effect—especially in colorectal cancer—other investigations have identified no appreciable advantage. The diversity in these results emphasizes the necessity of more extensive, long-term research to unequivocally prove the function of celecoxib in the prevention of cancer.
Future systematic reviews and meta-analyses as research develops will probably center on celecoxib’s effectiveness in fresh uses including mood disorders and antimicrobial treatment as well as the long-term safety and efficacy of new formulations and delivery methods. These continuous studies will be very vital in helping us to better grasp the position of celecoxib in contemporary medicine and direct treatment decisions.
Analysis of the Research Study “Celecoxib-Loaded Cubosomal Nanoparticles as a Therapeutic Approach for Staphylococcus aureus In Vivo Infection”
This study, conducted by Samar Zuhair Alshawwa, Thanaa A. El-Masry, Mohamed Nasr, Ahmed Y. Kira, Hadil Faris Alotaibi, Al-Sayed Sallam, and Engy Elekhnawy, presents an innovative approach to repurposing celecoxib for antibacterial therapy. Published in Microorganisms in 2023, this research explores the potential of nanoparticle-based drug delivery systems to enhance celecoxib’s efficacy against Staphylococcus aureus infections.
Methodology and Key Findings
Nanoparticle Formulation
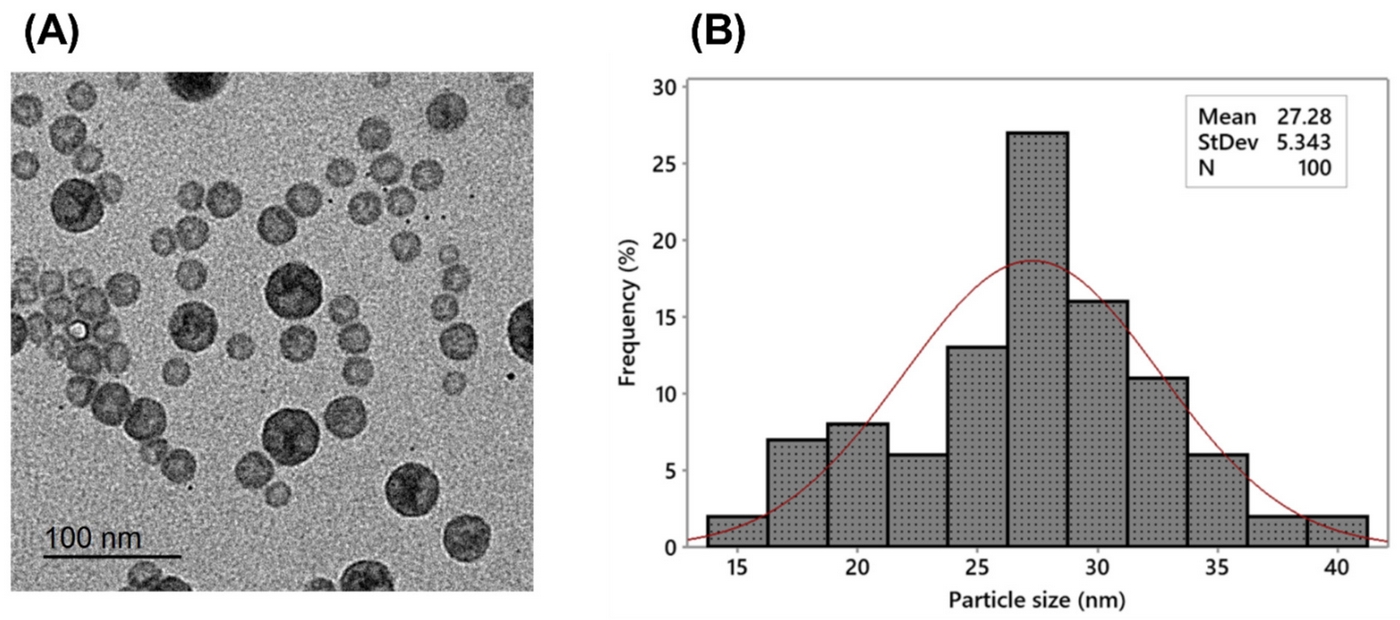
The researchers developed celecoxib-loaded cubosomal nanoparticles (Cel@Cubs) using glyceryl monooleate and Poloxamer 407. These nanoparticles demonstrated:
- A mean particle size of 128.15 ± 3.04 nm
- A polydispersity index of 0.235 ± 0.023
- A negative zeta potential of -17.50 ± 0.45 mV
- An entrapment efficiency of 88.57 ± 2.36%
These characteristics suggest a stable and uniform nanoparticle formulation with high drug loading capacity.
In Vitro Studies
The in vitro release profile of Cel@Cubs showed a biphasic pattern with an initial burst release followed by sustained release. The formulation exhibited antibacterial activity against S. aureus, with a minimum inhibitory concentration (MIC) of 16 μg/mL, significantly lower than that of free celecoxib (256 μg/mL).
In Vivo Efficacy
The in vivo studies using a mouse model of systemic S. aureus infection revealed:
- Reduced bacterial load in liver and spleen tissues
- Improved histological features in treated mice
- Decreased expression of inflammatory markers (IL-1β, IL-6, COX-2, TNF-α)
- Downregulation of NF-κB and caspase-3 expression in liver and spleen tissues
Critical Analysis
Strengths
- Novel Approach: The study presents an innovative method for repurposing celecoxib, potentially expanding its therapeutic applications.
- Comprehensive Evaluation: The research combines in vitro and in vivo studies, providing a thorough assessment of the formulation’s efficacy.
- Multifaceted Analysis: The use of various techniques (histological, immunohistochemical, molecular) offers a comprehensive view of the treatment’s effects.
Limitations
- Long-term Effects: The study does not address the long-term safety and efficacy of the nanoparticle formulation.
- Comparative Analysis: A direct comparison with conventional antibiotics would have provided more context for the formulation’s potential clinical value.
- Mechanism of Action: While the study demonstrates efficacy, it does not fully elucidate the mechanism by which celecoxib exerts its antibacterial effects.
Implications and Future Directions
This research opens new avenues for combating antibiotic-resistant bacteria using repurposed drugs. The success of celecoxib-loaded cubosomal nanoparticles against S. aureus suggests potential applications in treating other bacterial infections.
Future research directions could include:
- Investigating the formulation’s efficacy against a broader range of bacterial species
- Exploring combination therapies with conventional antibiotics
- Conducting long-term safety studies
- Elucidating the precise mechanisms of celecoxib’s antibacterial action in this nanoparticle form
Conclusion
Alshawwa et al.’s work offers a feasible method for improving the antibacterial qualities of celecoxib by means of nanoparticle composition. This discovery greatly advances the area of medication repurposing and nanoparticle-based antimicrobial treatments even although further study is required to completely grasp its possibilities and constraints. The results imply that, given the right dosage, celecoxib may help to solve the rising problem of antibiotic resistance.
Briefly
Celecoxib is a nonsteroidal anti-inflammatory drug (NSAID) that selectively inhibits cyclooxygenase-2 (COX-2). Among the many diseases including osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, it is mostly utilized to alleviate pain and inflammation related to each one. Its possible uses in mental disorders, cancer prevention, and antibiotic treatment have lately attracted study. Although typically well-tolerated, celecoxib may have adverse effects and must be used carefully considering personal patient circumstances for safety and effectiveness.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Alshawwa, Samar Zuhair, et al. “Celecoxib-Loaded Cubosomal Nanoparticles as a Therapeutic Approach for Staphylococcus aureus In Vivo Infection.” Microorganisms, vol. 11, no. 9, 2023, p. 2247. www.mdpi.com
- Bąk, Urszula, and Anna Krupa. “Challenges and Opportunities for Celecoxib Repurposing.” Pharmaceutical Research, vol. 40, 2023, pp. 2329–2345. springer.com
- Chen, Ruijie, et al. “Dual-targeting celecoxib nanoparticles protect intestinal epithelium and regulate macrophage polarization for ulcerative colitis treatment.” Chemical Engineering Journal, vol. 452, part 4, 2023, p. 139445. sciencedirect.com
- Cruz, J. V., et al. “The role of celecoxib as a potential inhibitor in the treatment of inflammatory diseases-A review.” Current Medicinal Chemistry, vol. 29, no. 17, 2022. ingentaconnect.com
- Gędek, Adam, et al. “Celecoxib for Mood Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.” Journal of Clinical Medicine, vol. 12, no. 10, 2023, p. 3497. www.mdpi.com
FAQ
What are the common celecoxib side effects?
Common celecoxib side effects include gastrointestinal issues such as nausea, diarrhea, and stomach pain. Some users may experience headaches, dizziness, or insomnia. Fluid retention and edema can also occur. It's crucial to consult a healthcare provider if any side effects persist or worsen, as individual responses may vary.
What is celecoxib 200 mg used for?
Celecoxib 200 mg is primarily prescribed for managing pain and inflammation associated with osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. It may also be used for acute pain relief and menstrual cramps. Always follow your doctor's instructions regarding dosage and duration of use, as individual treatment plans may vary.
Is celecoxib 200 mg a strong painkiller?
Celecoxib 200 mg is considered a moderately strong painkiller. Its effectiveness can vary depending on the individual and the condition being treated. While it's potent for many users, some may require different doses or alternative medications. Always consult your healthcare provider to determine the most appropriate pain management strategy for your specific needs.
Why was celecoxib banned in some countries?
Celecoxib wasn't universally banned, but concerns arose due to cardiovascular risks associated with COX-2 inhibitors. Some countries temporarily suspended its use or implemented stricter regulations. However, after further research and risk-benefit analysis, many reintroduced it with updated safety guidelines. Always follow your country's current regulations and medical advice regarding its use.
What are the potential celecoxib interactions?
Celecoxib may interact with various medications, including blood thinners, certain antidepressants, and some blood pressure medications. It can also interact with aspirin and other NSAIDs. These interactions can potentially increase the risk of side effects or alter the effectiveness of the medications. Always inform your healthcare provider about all medications you're taking to avoid potential interactions.
What is the difference between celecoxib 100 mg and 200 mg?
The primary difference lies in the dosage strength. Celecoxib 100 mg is often prescribed for milder conditions or as a starting dose, while 200 mg is typically used for more severe pain or inflammation. The appropriate dosage depends on the specific condition being treated and individual factors. Always follow your doctor's prescription and never adjust the dose without medical guidance.
Is celecoxib safe for long-term use?
Long-term use of celecoxib should be carefully monitored by a healthcare professional. While it can be effective for chronic conditions, prolonged use may increase the risk of certain side effects, particularly cardiovascular events. Regular check-ups and potential dose adjustments may be necessary. Always discuss the benefits and risks of long-term use with your doctor.


