Table of Contents
Toggle
Generic Name: Ebastine
Brand Names: Various around the world
What is Ebastine?
Ebastine is a second-generation antihistamine medication used to treat allergic rhinitis and chronic urticaria. Its side effects and uses will be extensively analysed later in this text. Discovered in the 1980s by the Spanish pharmaceutical company Almirall, ebastine was developed as a more selective and less sedating alternative to first-generation antihistamines. In this article, we will examine several medical journals and research papers that have investigated the efficacy, safety, and pharmacological properties of ebastine, focusing on the active ingredient rather than specific brand names. These studies provide valuable insights into the therapeutic potential and adverse effects associated with this antihistamine medication.
Chemical Structure and Mechanism of Action
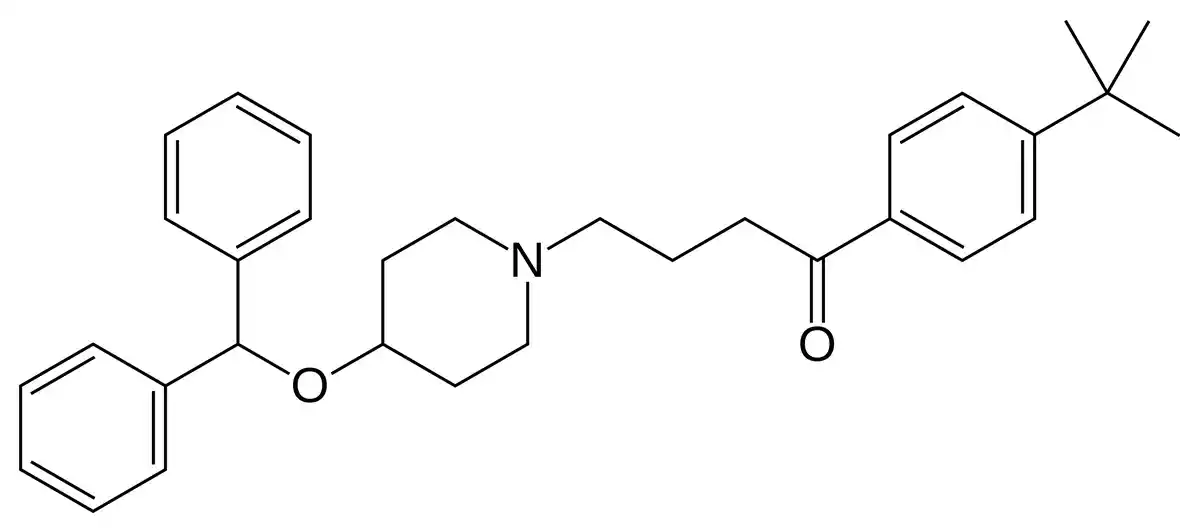
Ebastine, chemically known as 4-(4-benzhydryloxy-1-piperidyl)-1-(4-tert-butylphenyl)butan-1-one, is a long-acting, selective histamine H1 receptor antagonist. Its chemical structure consists of a piperidine ring linked to a diphenylmethoxy group and a 4-tert-butylphenyl ketone moiety (Sastre, 2020). Upon oral administration, ebastine undergoes extensive first-pass metabolism in the liver, primarily by the cytochrome P450 3A4 enzyme system, to form its active metabolite, carebastine (Ciprandi, 2010).
Carebastine is responsible for the majority of ebastine’s antihistaminic effects. It binds selectively and with high affinity to peripheral H1 receptors, preventing histamine from initiating the allergic response cascade. By inhibiting the action of histamine, carebastine effectively reduces symptoms such as sneezing, itching, rhinorrhoea, and urticaria associated with allergic conditions (Ohyama et al., 2010).
In addition to its antihistaminic properties, ebastine has been shown to exhibit anti-inflammatory and anti-allergic effects. It can inhibit the release of inflammatory mediators, such as leukotrienes and cytokines, from mast cells and basophils (Goyal et al., 2017). This additional mechanism of action may contribute to ebastine’s efficacy in managing allergic diseases.
Notably, ebastine has a low potential for central nervous system penetration due to its physicochemical properties and extensive metabolism. This characteristic minimizes the risk of sedation and cognitive impairment, which are common side effects associated with first-generation antihistamines (Haraguchi et al., 2014). The reduced central nervous system effects of ebastine make it a favourable option for patients seeking relief from allergic symptoms without experiencing significant drowsiness or impaired performance.
Uses
Ebastine is primarily indicated for the treatment of various allergic conditions, particularly allergic rhinitis and chronic urticaria:
- Allergic Rhinitis: Ebastine effectively manages the symptoms of allergic rhinitis, such as sneezing, nasal congestion, rhinorrhoea, and itching. Its efficacy in relieving these symptoms has been demonstrated in numerous clinical trials and real-world studies (Sastre). By antagonizing the effects of histamine at H1 receptors, ebastine reduces the inflammatory response triggered by allergens in the nasal mucosa.
- Chronic Urticaria: Ebastine is also used in the management of chronic urticaria, a condition characterized by the recurrent appearance of itchy, raised wheals on the skin. It helps alleviate the itching and reduces the frequency and severity of urticarial episodes. Goyal et al. conducted a comparative study evaluating the efficacy and safety of ebastine in acute urticaria, confirming its effectiveness in controlling symptoms.
In addition to its primary indications, ebastine has been explored for potential use in other conditions:
- Alopecia Areata: Ohyama et al. investigated the use of ebastine as a supportive medication for alopecia areata, an autoimmune disorder that causes patchy hair loss. Their experimental evaluation suggested that ebastine may have beneficial effects in managing this condition, although further research is needed to confirm its efficacy.
- Atopic Dermatitis: Given its anti-inflammatory properties, ebastine has been considered as a potential treatment option for atopic dermatitis, a chronic inflammatory skin disorder. While some studies have reported improvements in symptoms with ebastine use, more robust clinical trials are required to establish its effectiveness in this context.
Ebastine is available in various formulations, including tablets, orally disintegrating tablets (ODTs), and syrups. The development of ODTs has aimed to improve patient convenience and adherence. Haraguchi et al. evaluated the efficacy and safety of ebastine-loaded ODTs using a novel disintegration time detection apparatus and an electronic tongue system, highlighting the potential of this formulation to enhance patient experience.
The recommended dosage of ebastine for adults and children over 12 years is typically 10-20 mg once daily, depending on the severity of symptoms and individual response. Lower doses may be appropriate for certain populations, such as elderly patients or those with hepatic impairment.
Ebastine Side Effects
While ebastine is generally well-tolerated, it is essential for patients to be aware of the potential side effects associated with its use. The following sections outline the common, rare, and serious side effects that may occur during treatment with ebastine.
Common Ebastine Side Effects
The most frequently reported side effects of ebastine are typically mild and transient. These may include:
- Headache
- Drowsiness or fatigue
- Dry mouth
- Nausea or abdominal discomfort
- Dizziness or lightheadedness
In clinical studies and real-world experience, the incidence of these common side effects has been relatively low, with most patients tolerating ebastine well (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
Rare but Possible Ebastine Side Effects
Some less common side effects of ebastine have been reported in a small percentage of patients. These may include:
- Insomnia or sleep disturbances
- Nervousness or agitation
- Palpitations or rapid heartbeat
- Skin rash or pruritus
- Gastrointestinal disturbances, such as diarrhoea or constipation
If any of these side effects persist or worsen, patients should consult their healthcare provider for guidance.
Serious Ebastine Side Effects
In rare instances, ebastine may cause severe side effects that require immediate medical attention. These serious side effects may include:
- Allergic reactions, such as anaphylaxis or angioedema (swelling of the face, tongue, or throat)
- Severe skin reactions, such as Stevens-Johnson syndrome or toxic epidermal necrolysis
- Hepatic dysfunction or liver injury
- Seizures or convulsions
Patients should seek emergency medical care if they experience any signs or symptoms of these serious side effects.
It is important to note that the overall safety profile of ebastine is favourable, with a low incidence of serious adverse events. Ciprandi emphasizes that ebastine has demonstrated a high degree of patient adherence, which may be attributed to its good tolerability and low risk of sedation compared to other antihistamines.
However, as with any medication, individual responses may vary, and some patients may be more susceptible to certain side effects. Patients should discuss their medical history and any concerns with their healthcare provider before initiating treatment with ebastine. Regular monitoring and follow-up appointments can help identify and manage any potential adverse reactions.
By understanding the potential side effects associated with ebastine, patients can make informed decisions about their treatment and promptly report any concerning symptoms to their healthcare team.
Warnings
Before starting treatment with ebastine, patients should be aware of certain precautions and warnings to ensure safe and effective use:
- Allergic Reactions: Patients with a known hypersensitivity to ebastine or any of its components should not take this medication. Signs of an allergic reaction, such as rash, itching, difficulty breathing, or swelling of the face, tongue, or throat, require immediate medical attention.
- Hepatic Impairment: Ebastine undergoes extensive first-pass metabolism in the liver. Patients with severe hepatic impairment may require dosage adjustments or alternative treatments. Consulting with a healthcare provider is essential to determine the appropriate course of action (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
- Renal Impairment: While ebastine is primarily metabolized in the liver, patients with severe renal impairment should exercise caution. Dose reduction may be necessary, and close monitoring for adverse effects is recommended.
- Cardiovascular Disease: Ebastine, like other antihistamines, may cause slight increases in QT interval. Patients with pre-existing cardiac conditions, such as QT prolongation or a history of arrhythmias, should use ebastine with caution and under medical supervision.
- Elderly Patients: Older adults may be more sensitive to the effects of ebastine, particularly in terms of sedation and anticholinergic side effects. Lower initial doses and careful titration may be necessary to minimize the risk of adverse reactions.
- Pregnancy and Lactation: The safety of ebastine during pregnancy has not been definitively established. Pregnant women should only use ebastine if the potential benefits outweigh the risks. Consultation with a healthcare provider is essential to make an informed decision. It is unknown whether ebastine is excreted in human milk, so caution should be exercised when administering the drug to nursing mothers.
- Drug Interactions: Ebastine may interact with other medications, particularly those metabolized by the cytochrome P450 3A4 enzyme system. Patients should inform their healthcare provider about all medications, supplements, and herbal products they are taking to avoid potential drug interactions. Concomitant use of ebastine with strong CYP3A4 inhibitors, such as ketoconazole or erythromycin, may increase the risk of adverse effects (Haraguchi et al., “Evaluation of Ebastine-Loaded Orally Disintegrating Tablets Using New Apparatus of Detecting Disintegration Time and E-Tongue System”).
- Operating Machinery or Driving: Although ebastine has a low potential for causing sedation, some patients may experience drowsiness or impaired alertness. Patients should exercise caution when engaging in activities that require mental alertness, such as driving or operating machinery, until they know how ebastine affects them.
Precautions
When considering treatment with ebastine, several precautions should be taken into account to ensure safe and appropriate use:
- Patients with a history of hypersensitivity reactions to antihistamines should exercise caution when using ebastine. If any signs of allergic reaction occur, such as rash, itching, or difficulty breathing, the medication should be discontinued immediately, and medical attention should be sought.
- Ebastine is extensively metabolized in the liver by the cytochrome P450 3A4 enzyme system. Patients with hepatic impairment may require dosage adjustments or alternative treatments, as the pharmacokinetics of ebastine can be altered in these individuals (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
- Patients with severe renal impairment should use ebastine cautiously, as limited data exist regarding its safety and efficacy in this population. Dose reduction and close monitoring for adverse effects may be necessary.
- Ebastine should be used with caution in patients with pre-existing cardiovascular conditions, such as prolonged QT interval or a history of arrhythmias. Monitoring of the QT interval may be recommended in these patients.
- The safety of ebastine during pregnancy has not been conclusively established. Pregnant women should only use ebastine if the potential benefits justify the potential risks to the foetus. Consultation with a healthcare provider is crucial to make an informed decision.
Contraindications
In certain clinical situations, the use of ebastine is contraindicated due to the potential for serious adverse reactions or lack of safety data:
- Ebastine is contraindicated in patients with a known hypersensitivity to the drug or any of its components. Patients who have experienced allergic reactions to ebastine in the past should not take the medication again.
- The concomitant use of ebastine with strong CYP3A4 inhibitors, such as ketoconazole or erythromycin, is contraindicated. These medications can significantly increase the plasma concentration of ebastine, leading to an increased risk of adverse effects (Haraguchi et al., “Evaluation of Ebastine-Loaded Orally Disintegrating Tablets Using New Apparatus of Detecting Disintegration Time and E-Tongue System”).
- Ebastine is not recommended for use in children under the age of 12 years due to limited safety and efficacy data in this age group. The appropriate dosage and potential risks have not been definitively established in younger children.
- Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take ebastine oral lyophilisates, as they may contain lactose.
By carefully considering these precautions and contraindications, healthcare providers can make informed decisions regarding the suitability of ebastine for individual patients, minimizing the risk of adverse reactions and ensuring optimal treatment outcomes.
Interactions
Ebastine has the potential to interact with other medications, affecting their pharmacokinetics or pharmacodynamics. Some notable drug interactions include:
- CYP3A4 Inhibitors: Ebastine is metabolized by the cytochrome P450 3A4 enzyme system. Co-administration of ebastine with strong CYP3A4 inhibitors, such as ketoconazole, itraconazole, or erythromycin, can significantly increase ebastine plasma concentrations, leading to an increased risk of adverse effects (Haraguchi et al., “Evaluation of Ebastine-Loaded Orally Disintegrating Tablets Using New Apparatus of Detecting Disintegration Time and E-Tongue System”).
- CYP3A4 Inducers: Medications that induce CYP3A4 activity, such as rifampicin or phenytoin, may decrease the plasma concentrations of ebastine, potentially reducing its therapeutic efficacy.
- Antacids: The concomitant use of ebastine with antacids containing aluminium or magnesium hydroxide can decrease the absorption of ebastine, resulting in lower plasma concentrations. It is recommended to separate the administration of ebastine and antacids by at least 2 hours.
- CNS Depressants: Ebastine may cause drowsiness in some patients. The concurrent use of ebastine with other CNS depressants, such as alcohol, benzodiazepines, or opioids, may potentiate the sedative effects and impair alertness and coordination (Ciprandi).
- QT-Prolonging Medications: Ebastine may cause a slight increase in the QT interval. Caution should be exercised when using ebastine with other medications known to prolong the QT interval, such as certain antiarrhythmics, antipsychotics, or antibiotics.
Overdose
In the event of an ebastine overdose, patients may experience an exacerbation of the known side effects. Symptoms of an overdose may include:
- Drowsiness or sedation
- Tachycardia or palpitations
- Dry mouth
- Gastrointestinal disturbances, such as nausea or vomiting
- Headache or dizziness
- Agitation or restlessness
When an overdose of ebastine occurs in extreme circumstances, QT prolongation may occur, raising the possibility of cardiac arrhythmias such torsades de pointes.
Supportive care and symptomatic therapy are the mainstays of managing an ebastine overdose. If the overdose is discovered early, patients may be evaluated for gastric lavage or administration of activated charcoal. It is important to monitor vital signs, ECG, and electrolyte levels in order to identify and address any possible problems.
If a patient thinks they may have overdosed on ebastine, they should get help right away from a poison control centre. Since there isn’t a particular antidote for ebastine overdose, supportive care measures that are taken quickly and appropriately are crucial (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
Given the possible severity of an overdose on ebastine, patients should be counselled to closely follow the dosage recommendations and to store the drug out of the reach of minors to avoid inadvertent consumption.
Additional important information
Development of resilience
Ebastine, a second-generation antihistamine, has demonstrated a remarkable ability to withstand the test of time, maintaining its efficacy and safety profile over the course of its 30-year history. Since its initial development in the 1980s, ebastine has undergone extensive preclinical and clinical testing, establishing itself as a reliable and well-tolerated treatment option for allergic rhinitis and chronic urticaria (Sastre).
The resilience of ebastine can be attributed to its unique pharmacological properties, which strike a balance between potent antihistaminic activity and minimal central nervous system penetration. This characteristic has allowed ebastine to provide effective symptom relief while minimizing the risk of sedation and cognitive impairment, which are commonly associated with first-generation antihistamines (Ciprandi).
Preclinical and Clinical Studies
An extensive programme of preclinical and clinical research helped to create ebastine. Preclinical research examined the pharmacologic and pharmacokinetic characteristics of ebastine, determining its safety profile and selectivity for peripheral H1 receptors. Additionally, these investigations clarified the metabolic pathway of ebastine, determining that the main source of its antihistaminic actions is carebastine, the drug’s active metabolite (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
Ebastine has proven to be effective in treating both chronic urticaria and allergic rhinitis in subsequent clinical trials. In individuals with these allergic diseases, ebastine dramatically improves pruritus, wheals, and nasal and ocular symptoms, according to randomised, double-blind, placebo-controlled trials. Furthermore, comparative studies have shown that ebastine has a better tolerability profile and is just as effective as other second-generation antihistamines like loratadine and cetirizine (Goyal et al., “Comparative Efficacy and Safety of Ebastine 20 mg, Ebastine 10 mg, and Levocetirizine 5 mg in Acute Urticaria”).
Post-approval studies, Pharmacovigilance and Pharmacokinetic characteristics
Ever since its first clearance, ebastine has been the focus of continuous pharmacovigilance and post-marketing surveillance. These investigations have shed important light on the long-term efficacy and safety of ebastine in actual clinical settings. Studies conducted after ebastine’s clearance have verified its long-term effectiveness and low rate of side effects (Sastre).
QT prolongation and hepatic dysfunction are two uncommon but possible side effects of ebastine usage that have been made clear by pharmacovigilance data. The safe use of ebastine in a variety of patient demographics has been facilitated by these findings, which have also helped to improve prescribing recommendations (Haraguchi et al.).
Ebastine’s pharmacokinetic properties have been thoroughly investigated, offering a comprehensive comprehension of its absorption, distribution, metabolism, and excretion. Following quick absorption from the digestive system, ebastine is extensively first-pass metabolised in the liver, mostly via the CYP3A4 enzyme system. Because carebastine, the ensuing active metabolite, has a half-life of around 15–19 hours, it can be taken once daily (Sastre).
The body of information gathered from pharmacokinetic analysis, post-approval surveillance, and preclinical and clinical trials has strengthened ebastine’s standing as a beneficial treatment choice for individuals with allergic disorders. With the use of this extensive body of data, medical professionals may optimise treatment outcomes and reduce the risk of side effects by making well-informed decisions about the use of ebastine in clinical practice.
Comparative effectiveness
The medical community has shown a great deal of interest in ebastine’s relative efficacy to other second-generation antihistamines. Comparing ebastine to medications like cetirizine, loratadine, and fexofenadine, many studies have attempted to assess the relative effectiveness and safety of ebastine.
Goyal et al. compared the safety and effectiveness of ebastine 10 mg, ebastine 20 mg, and levocetirizine 5 mg in patients with acute urticaria in a randomised, double-blind, placebo-controlled research. The outcomes showed that ebastine, at both dosages, had a similar safety profile and was just as successful in lowering urticaria symptoms as levocetirizine. In treating acute urticaria, ebastine is not less effective than other well-known antihistamines, as this research (“Comparative Efficacy and Safety of Ebastine 20 mg, Ebastine 10 mg, and Levocetirizine 5 mg in Acute Urticaria”) demonstrates.
Similarly, the effectiveness and tolerability of cetirizine and ebastine in the treatment of persistent allergic rhinitis were evaluated in a multicenter, randomised, double-blind research. According to the study, ebastine improved nasal symptoms and quality of life just as well as cetirizine did, with a decreased risk of sedation and weariness (Sastre).
Systematic reviews and meta-analyses
The synthesis of the existing data on the relative efficacy and safety of ebastine has been greatly aided by systematic reviews and meta-analyses. By pooling data from several clinical trials, these research have used rigorous procedures to provide a more thorough and trustworthy evaluation of ebastine’s performance.
Ciprandi et al. conducted a comprehensive review and meta-analysis to evaluate the safety and effectiveness of ebastine in the management of allergic rhinitis. A total of 2,845 patients’ data from 10 randomised controlled trials were included in the study. According to the findings, ebastine considerably outperformed a placebo in terms of easing nasal symptoms and enhancing quality of life. Additionally, the meta-analysis supported ebastine’s good safety profile, which includes a low frequency of sedation and other side effects.
The comparative effectiveness and safety of second-generation antihistamines, such as ebastine, in the management of chronic urticaria were the subject of another systematic study. Ten randomised controlled studies that directly assessed the effectiveness and safety of various antihistamines were found throughout the study. According to the analysis, ebastine had a similar safety profile and was just as successful in lowering urticaria symptoms as other second-generation antihistamines (Sastre, “Ebastine in the Treatment of Allergic Rhinitis and Urticaria: 30 Years of Clinical Studies and Real-World Experience”).
Current research directions and future perspectives
Even with the three decades of intensive study on ebastine, there are still a number of topics that need to be investigated further. The possible use of ebastine in the treatment of inflammatory and allergic diseases other than urticaria and allergic rhinitis is the subject of one ongoing study line. Ohyama et al., for instance, looked at the potential of ebastine as a supportive treatment for autoimmune diseases such alopecia areata, which result in patchy hair loss. Notwithstanding the encouraging outcomes, more investigation is required to verify ebastine’s effectiveness in this particular situation (“Experimental Evaluation of Ebastine, a Second-Generation Anti-Histamine, as a Supportive Medication for Alopecia Areata”).
The creation of innovative ebastine medication delivery methods and formulations is another topic of focus. Haraguchi et al. used an electronic tongue system and a novel device to measure the disintegration time in order to assess the safety and effectiveness of ebastine-loaded oral disintegrating tablets. By offering a quickly dissolving tablet that may be taken without water, this novel formulation seeks to improve patient convenience and adherence (“Evaluation of Ebastine-Loaded Orally Disintegrating Tablets Using New Apparatus of Detecting Disintegration Time and E-Tongue System”).
Subsequent investigations might explore the possible synergistic impacts of ebastine in combination with other pharmaceuticals or non-pharmacological therapies. Furthermore, long-term research is necessary to evaluate the safety and long-term effectiveness of ebastine, especially in those with chronic allergy disorders.
The function of ebastine in the treatment of allergic disorders may develop or change as our knowledge of the pathophysiology of these illnesses advances. The position of ebastine in the dynamic field of allergy therapy will be shaped by ongoing research and emerging viewpoints, guaranteeing that patients will have access to safe, efficient, and scientifically supported treatment alternatives.
Briefly
Ebastine is a second-generation antihistamine medication used to treat allergic rhinitis and chronic urticaria. It functions by specifically opposing peripheral H1 receptors, which stops histamine from doing its job and relieves symptoms including sneezing, itching, and skin rashes. Ebastine exhibits a favourable safety profile marked by low sedation and cognitive impairment, and it has showed effectiveness equivalent to other second-generation antihistamines. Its once-daily dosage is made possible by its pharmacokinetic characteristics, which include quick absorption and thorough metabolism to the active metabolite carebastine. Clinical and preclinical research have proven ebastine’s efficacy and tolerability, and post-marketing surveillance has attested to its long-term safety. Current studies are investigating ebastine’s possible use in additional inflammatory and allergy disorders as well as the creation of innovative formulations to improve patient adherence. Ebastine continues to be a useful treatment choice as our understanding of allergic disorders advances, offering patients efficient and well-tolerated relief from the burden of allergy symptoms.
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient rather than specific brand names containing this generic medicine worldwide. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Sastre, J. (2020). Ebastine in the treatment of allergic rhinitis and urticaria: 30 years of clinical studies and real-world experience. Journal of Investigational Allergology and Clinical Immunology, 30(3), 156-168. jiaci
- Ciprandi, G. (2010). Clinical utility and patient adherence with ebastine for allergic rhinitis. Patient Preference and Adherence, 4, 389-393. tandfonline
- Ohyama, M., Shimizu, A., Tanaka, K., & Ebihara, T. (2010). Experimental evaluation of ebastine, a second-generation anti-histamine, as a supportive medication for alopecia areata. Journal of Dermatological Science, 60(1), 50-54. jdsjournal
- Goyal, V., Gupta, A., Gupta, O., Lal, D., & Gill, M. (2017). Comparative efficacy and safety of ebastine 20 mg, ebastine 10 mg and levocetirizine 5 mg in acute urticaria. Journal of Clinical and Diagnostic Research, 11(5), WC06-WC09. ncbi
- Haraguchi, T., Yoshida, M., & Uchida, T. (2014). Evaluation of ebastine-loaded orally disintegrating tablets using new apparatus of detecting disintegration time and e-tongue system. Journal of Drug Delivery Science and Technology, 24(6), 684-688. sciencedirect