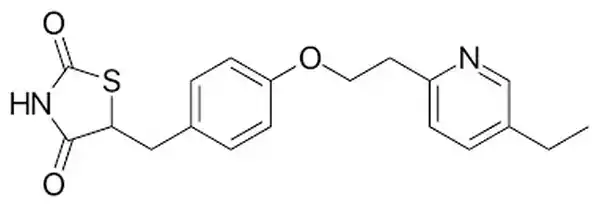
Generic Name: Pioglitazone
Brand Names: Various around the world
Drug Class: Thiazolidinedione antidiabetic agent
Pioglitazone Side Effects, Uses, Dosage, and More
What is pioglitazone?
Pioglitazone is an oral antidiabetic medication used to treat type 2 diabetes mellitus. It belongs to the thiazolidinedione class of drugs, which work by increasing insulin sensitivity in peripheral tissues. Pioglitazone is prescribed to improve glycemic control in adults with type 2 diabetes, either as monotherapy or in combination with other antidiabetic agents like metformin or sulfonylureas. It may be used when diet, exercise, and other medications do not provide adequate blood glucose control.
Multiple pharmaceutical companies worldwide manufacture and distribute pioglitazone under various brand names. The drug is typically prescribed for patients whose blood sugar levels are not well-controlled by other medications or lifestyle modifications alone.
Mechanism of action: Pioglitazone activates peroxisome proliferator-activated receptor gamma (PPAR-γ), enhancing insulin sensitivity in muscle, fat, and liver cells.
Chemical structure: Contains a thiazolidinedione ring structure linked to an aromatic ring system.
Therapeutic category: Oral hypoglycemic agent, insulin sensitizer.
Anatomical/therapeutic/chemical (ATC) classification
ATC Code: A10BG03 Title: Pioglitazone Classification: A (Alimentary tract and metabolism) > A10 (Drugs used in diabetes) > A10B (Blood glucose lowering drugs, excl. insulins) > A10BG (Thiazolidinediones) > A10BG03 (Pioglitazone)
History of Medicine
Takeda Pharmaceuticals came across and developed pioglitazone in the 1990s. The U.S. Food and Drug Administration (FDA) approved it for use treating type 2 diabetes in 1999. The medication was brought in to meet the rising need for more potent treatments to regulate glycemic control and lower insulin resistance in diabetic patients. Pioglitazone has been extensively investigated and used worldwide since its approval, rising to be a major choice for diabetic treatment.
This article will analyze recent research on pioglitazone, including studies published in Molecular Biotechnology, Diabetes, Obesity and Metabolism, and ADCES in Practice. These publications provide insights into the drug’s efficacy, safety profile, and potential new applications.
Indications of pioglitazone
Pioglitazone is primarily indicated for the management of type 2 diabetes mellitus. It is prescribed to improve glycemic control in adult patients, particularly when other treatments have proven insufficient. This medication may be used:
- As monotherapy in patients inadequately controlled by diet and exercise alone
- In combination with metformin when metformin alone does not provide adequate glycemic control
- In combination with a sulfonylurea when sulfonylurea monotherapy is inadequate
- As an adjunct to insulin in patients with type 2 diabetes who are already receiving insulin treatment
Recent research has explored additional potential uses for pioglitazone. Bell and Jerkins note that the drug may have positive effects on cardiac risk factors and surrogate measures of cardiovascular disease, potentially lowering the incidence of cardiac events in diabetic patients (Diabetes, Obesity and Metabolism).
Contraindications and Precautions
Pioglitazone is contraindicated in patients with:
- Established or history of heart failure
- Hypersensitivity to the drug or any of its components
- Active bladder cancer or a history of bladder cancer
- Severe hepatic impairment
Precautions should be taken in patients with:
- Edema or fluid retention
- Macular edema
- Liver disease or elevated liver enzymes
- Bone fracture risk, especially in postmenopausal women
- Unexplained hematuria
Special warnings for the elderly, children and pregnant women
Elderly: Older adults may be more susceptible to fluid retention and heart failure. Dosage adjustments may be necessary.
Children: Safety and efficacy in pediatric patients have not been established. Pioglitazone is not recommended for use in children.
Pregnant women: Pioglitazone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women of childbearing age should use effective contraception while taking this medication.
Dosage and administration
The typical starting dose is 15 or 30 mg once daily, taken orally with or without food. The dose may be increased to 45 mg once daily if needed for glycemic control. Dosage adjustments should be made based on individual patient response and tolerability.
What should I do if I miss a dose?
If a dose is missed, it should be taken as soon as remembered. However, if it is almost time for the next scheduled dose, the missed dose should be skipped. Patients should not take a double dose to make up for a missed one.
Uses of pioglitazone
Beyond its primary use in type 2 diabetes management, Charbonneau and Capoccia highlight that pioglitazone may have potential benefits in other conditions associated with insulin resistance (ADCES in Practice). These include:
- Non-alcoholic fatty liver disease (NAFLD)
- Polycystic ovary syndrome (PCOS)
- Potential neuroprotective effects in certain neurological disorders
Overdose
Symptoms of overdose may include hypoglycemia, fluid retention, and edema. In case of overdose, supportive measures should be initiated, and the patient should be monitored closely. There is no specific antidote for pioglitazone overdose.
Interactions
Drug-drug interactions
- Gemfibrozil: May increase pioglitazone levels
- Rifampin: May decrease pioglitazone effectiveness
- CYP2C8 inhibitors: May increase pioglitazone exposure
- Oral contraceptives: Pioglitazone may decrease their effectiveness
Drug-food interactions
No significant food interactions have been reported with pioglitazone. However, patients should maintain a consistent diet while taking this medication to help manage blood glucose levels effectively.
Pioglitazone Side Effects
While pioglitazone can be an effective treatment for type 2 diabetes, it may cause various side effects. Understanding these potential adverse reactions is crucial for patients and healthcare providers. Common side effects of pioglitazone include:
- Weight gain
- Fluid retention and edema
- Upper respiratory tract infections
- Headache
- Muscle pain
Botrous et al. observed in their study that pioglitazone treatment induced hepatotoxicity, renal inflammation, and hematological disorders in male albino mice (Molecular Biotechnology). These findings underscore the importance of monitoring liver and kidney function in patients taking this medication.
Bell and Jerkins note that pioglitazone may cause fluid retention, which could potentially exacerbate or precipitate heart failure in susceptible individuals (Diabetes, Obesity and Metabolism). They emphasize the need for careful patient selection and monitoring when prescribing this medication.
Rare but Possible Pioglitazone Side Effects
While less common, some serious side effects may occur with pioglitazone use:
- Bladder cancer: Long-term use and high cumulative doses of pioglitazone have been associated with an increased risk of bladder cancer. Botrous et al. reported “moderate to severe dysplastic changes in the lining urothelium and moderate inflammatory infiltrates in the submucosa” in mice treated with pioglitazone (Molecular Biotechnology).
- Bone fractures: Particularly in postmenopausal women, pioglitazone may increase the risk of bone fractures.
- Macular edema: Some patients have reported blurred vision or decreased visual acuity due to macular edema.
- Hypoglycemia: Especially when used in combination with other antidiabetic medications.
- Liver toxicity: Rare cases of severe hepatotoxicity have been reported.
Charbonneau and Capoccia highlight that while pioglitazone has been associated with certain risks, its benefits in glycemic control and potential cardioprotective effects should be weighed against these potential adverse effects (ADCES in Practice).
How to Manage Pioglitazone Side Effects
Managing side effects of pioglitazone requires a collaborative approach between patients and healthcare providers:
- Regular monitoring: Patients should undergo regular liver function tests, particularly during the first year of treatment. Bladder cancer screening may be recommended for high-risk individuals.
- Dose adjustment: If side effects are severe, the healthcare provider may reduce the dose or consider alternative treatments.
- Lifestyle modifications: To counteract weight gain, patients may be advised to maintain a balanced diet and engage in regular physical activity.
- Fluid retention management: Patients experiencing edema may be instructed to limit salt intake and monitor their weight regularly. In some cases, diuretics may be prescribed.
- Bone health: For patients at risk of fractures, calcium and vitamin D supplementation may be recommended, along with bone density monitoring.
- Patient education: Patients should be informed about potential side effects and instructed to report any unusual symptoms promptly.
Bell and Jerkins propose that preclinical stages of heart failure’s diastolic function may be genuinely improved and progression to heart failure prevented by using pioglitazone (Diabetes, Obesity and Metabolism). This emphasizes the need of cautious patient choice and timing of pioglitazone starting.
Patients should be honest with their doctors about any negative effects they run into. Making decisions on whether to keep, change, or stop pioglitazone should be based on personal judgment weighing the possible hazards against the therapeutic advantages.
Additional Important Information of Pioglitazone
Resistance Development
Long-term diabetes treatment takes much thought on the evolution of resistance to pioglitazone. Although not as prevalent as some other antidiabetic drugs, resistance might develop with time and therefore lessen the effectiveness of the treatment. Often linked with type 2 diabetes’s characteristic of increasing beta-cell failure is this phenomena. As the condition advances, patients might need combination treatment or dosage changes to maintain glycemic control.
Preclinical and Clinical Studies
Important new perspectives on the mechanism of action and possible therapeutic uses of pioglitazone have come from preclinical investigations. The drug’s capacity to raise insulin sensitivity and glucose homeostasis has been shown by animal models Examining male albino mice, Botrous et al. investigated the effects of pioglitazone in concert with Artemisia annua L. extract. According to their results, concurrent therapy with both drugs might help to reduce some of pioglitazone’s side effects (Molecular Biotechnology).
Pioglitazone’s effectiveness and safety profile in human beings have been clarified even further by clinical investigations. Glycemic control and insulin sensitivity have usually showed significant increases in these investigations. They have also underlined possible hazards, including weight increase and fluid retention, which need close observation.
Post-authorization Studies and Pharmacovigilance
Identification of uncommon but significant side effects linked to pioglitazone usage has been much aided by post-marketing monitoring. Particularly with longer usage and larger cumulative dosages, long-term observational studies have generated questions about a higher risk of bladder cancer. Some nations, even those with limits on usage in certain patient categories, have responded to these results with legislative action.
With specific focus on cardiovascular outcomes, fracture risk, and possible cancer links, ongoing pharmacovigilance activities track the safety profile of pioglitazone. Maintaining a current benefit-risk evaluation of the drug depends on these initiatives.
Pharmacokinetic Characteristics of Pioglitazone
Pioglitazone exhibits favorable pharmacokinetic properties, contributing to its clinical utility. The drug is rapidly absorbed following oral administration, with peak plasma concentrations typically reached within two hours. It has a high volume of distribution and is extensively protein-bound in plasma. Pioglitazone undergoes hepatic metabolism, primarily via cytochrome P450 2C8 and 3A4 enzymes, resulting in several active metabolites that contribute to its pharmacological effects.
The elimination half-life of pioglitazone ranges from 3 to 7 hours, while its active metabolites have longer half-lives, contributing to the drug’s once-daily dosing regimen. Excretion occurs primarily via the fecal route, with a small proportion eliminated renally.
Current Research Directions and Future Perspectives
New uses of pioglitazone outside of diabetes control are being investigation right now. Bell and Jerkins stress possible advantages in non-alcoholic fatty liver disease treatment and prevention of cardiovascular disease (Diabetes, Obesity and Metabolism). Given pioglitazone’s possible neuroprotective effects, ongoing research is looking at its function in neurodegenerative diseases.
Targeting specific delivery mechanisms to improve effectiveness while lowering systemic adverse effects might be part of future avenues of study. Furthermore under investigation are combination treatments using newly developed antidiabetic drugs to maximize glycemic control and handle certain pathophysiological features of type 2 diabetes.
Effectiveness
Pioglitazone’s effectiveness in improving glycemic control is well-established. The drug typically reduces HbA1c levels by 0.5-1.4%, depending on baseline values and treatment duration. Its insulin-sensitizing effects can lead to improved lipid profiles and potential cardiovascular benefits. Charbonneau and Capoccia note that pioglitazone may have positive effects on beta-cell function, potentially slowing disease progression (ADCES in Practice).
Comparative Efficacy, Systematic Reviews and Meta-analyses
Comprehensive assessments of pioglitazone’s effectiveness in relation to other antidiabetic drugs have come from systematic reviews and meta-analyses. Generally speaking, these trials find pioglitazone to be a good second-line or add-on treatment for type 2 diabetes. Comparisons with other thiazolidinediones, including rosiglitazone, have revealed comparable glycemic effectiveness but different adverse effect profiles.
Meta-analyses have also looked at how pioglitazone affects cardiovascular outcomes; some have shown possible advantages in lowering the risk of severe adverse events. These results should be taken carefully, however, given the diversity of research populations and approaches.
In essence, even while pioglitazone is still a vital instrument for treating diabetes, its usage calls for careful evaluation of personal patient circumstances, possible advantages, and hazards. Constant research helps us to better grasp this complicated drug, thereby enhancing its safety profile and maybe extending its therapeutic uses.
Scientific Research
Analysis of the Research Study “Inhibition of TNF-α Oncogene Expression by Artemisia Annua L. Extract Against Pioglitazone Side Effects in Male Albino Mice”
This study, conducted by Silvia Botrous, Ayaat Elmaghraby, Samar El-Achy, Yehia Mustafa, Effat Badr, Amany Haggag, and Salah Abdel-Rahman, and published in Molecular Biotechnology in 2024, presents an intriguing investigation into potential mitigating strategies for pioglitazone side effects.
Study Objectives
The primary aim of this research was to evaluate the effectiveness of Artemisia annua L. extract in counteracting the adverse effects associated with pioglitazone use in male albino mice. The study focused on several key aspects:
- Hepatotoxicity assessment
- Renal inflammation evaluation
- Hematological disorders analysis
- Bladder cancer risk examination
Methodology
The researchers employed a comprehensive approach:
- Animal model: Male albino mice were used as the experimental subjects.
- Treatment groups: The study included various groups treated with pioglitazone alone, Artemisia annua extract alone, and combinations of both.
- Dosage: Pioglitazone was administered at 45 mg/kg, while Artemisia annua extract was given at 4 g/kg.
- Duration: The experiment was conducted over two time periods: 15 days and 30 days.
- Analysis methods:
- Biochemical and hematological tests
- Histopathological examinations of liver, kidney, testis, and urinary bladder tissues
- Quantitative Real-Time PCR for TNF-α gene expression in bladder tissues
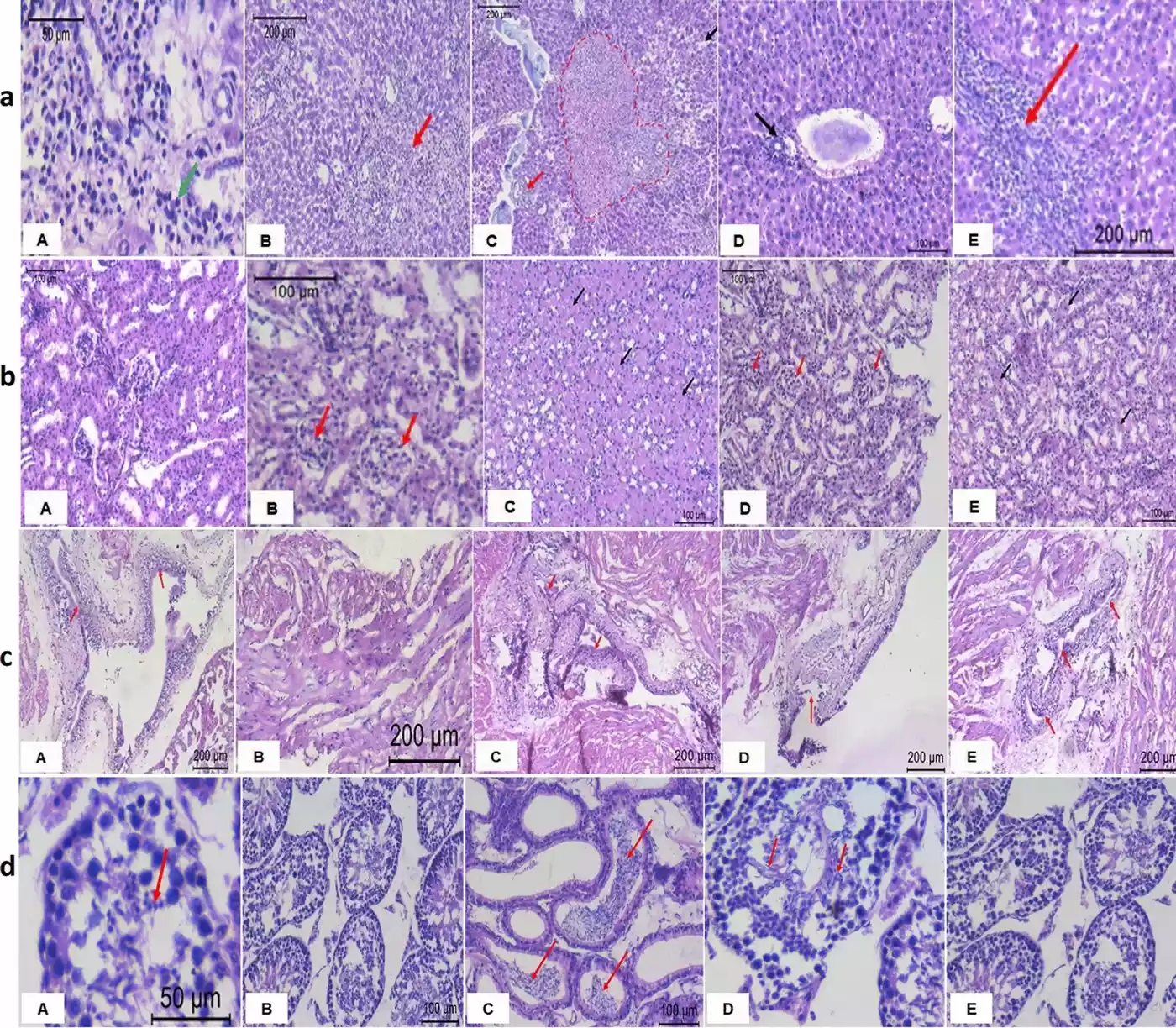
Key Findings
- Pioglitazone-induced toxicity:
- The study confirmed that pioglitazone alone induced hepatotoxicity, renal inflammation, and hematological disorders.
- Histopathological changes were observed in liver and kidney tissues.
- Moderate to severe dysplastic changes were noted in the bladder urothelium.
- Artemisia annua extract effects:
- Concurrent treatment with pioglitazone and Artemisia annua extract showed significant improvements in biochemical, hematological, and histopathological parameters.
- The extract appeared to mitigate the harmful side effects of pioglitazone.
- TNF-α oncogene expression:
- A remarkable finding was the significant decrease (by about 99.99%) in TNF-α oncogene expression levels in bladder tissues when treated with the combination of pioglitazone and Artemisia annua extract.
Strengths of the Study
- Comprehensive approach: The research examined multiple physiological systems, providing a holistic view of pioglitazone’s effects and the potential benefits of Artemisia annua extract.
- Quantitative analysis: The use of qRT-PCR for TNF-α gene expression offered precise, quantifiable data.
- Time-course examination: By conducting the experiment over both 15 and 30-day periods, the researchers could observe both short-term and slightly longer-term effects.
Limitations and Considerations
- Animal model: While mice models are valuable, extrapolation to human physiology requires caution.
- Gender specificity: The study focused on male mice, leaving questions about potential gender-specific effects unanswered.
- Mechanism of action: While the study demonstrated effects, it did not fully elucidate the molecular mechanisms by which Artemisia annua extract mitigates pioglitazone side effects.
Implications and Future Directions
This research opens up exciting possibilities for managing pioglitazone side effects. The significant reduction in TNF-α oncogene expression is particularly noteworthy, given the role of TNF-α in inflammation and cancer development. Future studies could:
- Investigate the specific compounds in Artemisia annua extract responsible for the observed effects.
- Explore potential synergistic effects between pioglitazone and Artemisia annua extract in diabetes management.
- Conduct longer-term studies to assess the safety and efficacy of this combination approach.
- Extend the research to female subjects and eventually to human clinical trials.
In summary, even if this study offers encouraging findings for reducing pioglitazone side effects, further research is required before therapeutic uses may be regarded. Correctly stressing that “for application more studies must be achieved in that field,” the writers Still, this study marks a major step towards maybe improving the safety profile of a crucial antidiabetic drug.
Briefly
Pioglitazone is an oral antidiabetic medication used to treat type 2 diabetes mellitus. It falls in the thiazolidinedione family of medications, which raise peripheral tissue insulin sensitivity. Usually, when other medications fall short in providing sufficient glycemic control, pioglitazone is advised. Although it helps some people control blood glucose levels, it may have negative effects, including weight gain, fluid retention, and a higher risk of heart failure. Combining pioglitazone with natural extracts is one of the most recently investigated possible ways to reduce these negative effects.
enofmedicines.com
ATTENTION: It is crucial never to take medication without a qualified doctor’s supervision. Always read the Patient Information Leaflet (PIL) with each prescribed medicine. Pharmaceutical companies accurately describe each product’s details, which may be regularly updated, though variations may exist depending on the drug’s composition. This article analyses the active ingredient/s rather than specific brand names containing this generic medicine. Study the instruction leaflet for each preparation you use. Close cooperation with your doctor and pharmacist is vital. Self-administering medication carries serious health risks and must be strictly avoided.
Bibliography
- Bell, David S.H., and Trenton Jerkins. “In praise of pioglitazone: An economically efficacious therapy for type 2 diabetes and other manifestations of the metabolic syndrome.” Diabetes, Obesity and Metabolism, 2023. onlinelibrary.wiley.com
- Botrous, Silvia, et al. “Inhibition of TNF-α Oncogene Expression by Artemisia Annua L. Extract Against Pioglitazone Side Effects in Male Albino Mice.” Molecular Biotechnology, vol. 66, 2024, pp. 432-441. link.springer.com
- Charbonneau, Monique S., and Kristina L. Capoccia. “Pioglitazone: Reviewed and renewed.” ADCES in Practice, 2022. journals.sagepub.com
FAQ
What are the common pioglitazone side effects related to weight gain?
Weight gain is a frequent side effect of pioglitazone, typically ranging from 2-5 kg. This occurs due to increased fat storage and fluid retention. Patients should monitor their weight regularly and consult their doctor if significant changes occur. A balanced diet and exercise may help manage this side effect. Always seek medical advice before making any changes to your treatment plan.
How does pioglitazone potentially affect liver function?
Pioglitazone can rarely cause liver problems. Symptoms may include nausea, vomiting, abdominal pain, fatigue, and jaundice. Regular liver function tests are recommended, especially during the first year of treatment. If you experience any unusual symptoms, contact your healthcare provider immediately. Never adjust your medication without professional medical guidance.
What are the concerns regarding pioglitazone side effects on the kidneys?
While pioglitazone is not directly toxic to the kidneys, it can cause fluid retention, which may impact kidney function. Patients with existing kidney issues should be monitored closely. Signs of kidney problems include swelling in extremities, changes in urination, and fatigue. Always inform your doctor about any pre-existing conditions and report new symptoms promptly.
Can pioglitazone cause fluid retention, and how is it managed?
Yes, fluid retention is a known side effect of pioglitazone. It can lead to swelling in the legs, ankles, and feet. In severe cases, it may contribute to heart failure. Management includes regular weight checks, limiting salt intake, and possibly using diuretics. Your doctor may adjust the dosage if fluid retention becomes problematic. Seek medical advice if you notice sudden weight gain or swelling.
What are the potential pioglitazone side effects on the heart?
Pioglitazone can increase the risk of heart failure, especially in patients with existing cardiovascular issues. Symptoms may include shortness of breath, rapid weight gain, and swelling. Regular cardiac check-ups are crucial for patients on pioglitazone. If you experience any chest pain or difficulty breathing, seek immediate medical attention. Your doctor will weigh the benefits against risks before prescribing.
Are there any specific pioglitazone side effects in elderly patients?
Elderly patients may be more susceptible to pioglitazone's side effects, particularly fluid retention and heart problems. They may also have an increased risk of bone fractures. Careful monitoring and possibly lower starting doses are often recommended for older adults. Regular check-ups and open communication with healthcare providers are essential. Never change your medication regimen without professional guidance.
Is there a connection between pioglitazone side effects and bladder cancer?
Some studies have suggested a potential link between long-term pioglitazone use and an increased risk of bladder cancer. However, the evidence is not conclusive. Patients should be aware of symptoms like blood in urine or painful urination. Regular urinary screenings may be recommended. Discuss any concerns with your doctor, who can help weigh the benefits and risks of treatment.


